Page 52 - Instant notes
P. 52
Physical Chemistry 38
of material doubles the mass. The internal energy is another example of an extensive
property. The value of an intensive property is independent of the amount of material
present. An example is the temperature or the density of a substance.
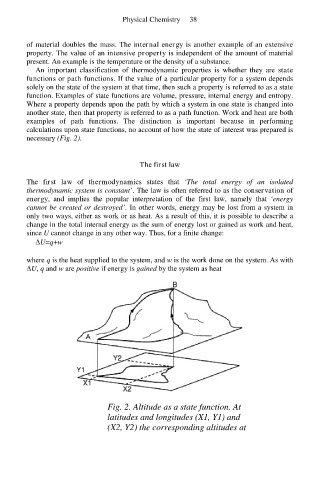
An important classification of thermodynamic properties is whether they are state
functions or path functions. If the value of a particular property for a system depends
solely on the state of the system at that time, then such a property is referred to as a state
function. Examples of state functions are volume, pressure, internal energy and entropy.
Where a property depends upon the path by which a system in one state is changed into
another state, then that property is referred to as a path function. Work and heat are both
examples of path functions. The distinction is important because in performing
calculations upon state functions, no account of how the state of interest was prepared is
necessary (Fig. 2).
The first law
The first law of thermodynamics states that ‘The total energy of an isolated
thermodynamic system is constant’. The law is often referred to as the conservation of
energy, and implies the popular interpretation of the first law, namely that ‘energy
cannot be created or destroyed’. In other words, energy may be lost from a system in
only two ways, either as work or as heat. As a result of this, it is possible to describe a
change in the total internal energy as the sum of energy lost or gained as work and heat,
since U cannot change in any other way. Thus, for a finite change:
∆U=q+w
where q is the heat supplied to the system, and w is the work done on the system. As with
∆U, q and w are positive if energy is gained by the system as heat
Fig. 2. Altitude as a state function. At
latitudes and longitudes (X1, Y1) and
(X2, Y2) the corresponding altitudes at