Page 80 - Instant notes
P. 80
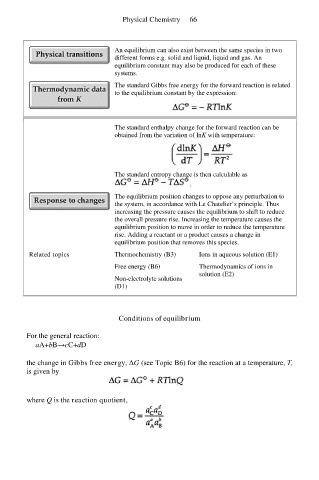
Physical Chemistry 66
An equilibrium can also exist between the same species in two
different forms e.g. solid and liquid, liquid and gas. An
equilibrium constant may also be produced for each of these
systems.
The standard Gibbs free energy for the forward reaction is related
to the equilibrium constant by the expression:
The standard enthalpy change for the forward reaction can be
obtained from the variation of lnK with temperature:
The standard entropy change is then calculable as
.
The equilibrium position changes to oppose any perturbation to
the system, in accordance with Le Chatelier’s principle. Thus
increasing the pressure causes the equilibrium to shift to reduce
the overall pressure rise. Increasing the temperature causes the
equilibrium position to move in order to reduce the temperature
rise. Adding a reactant or a product causes a change in
equilibrium position that removes this species.
Related topics Thermochemistry (B3) Ions in aqueous solution (E1)
Free energy (B6) Thermodynamics of ions in
solution (E2)
Non-electrolyte solutions
(D1)
Conditions of equilibrium
For the general reaction:
aA+bB→cC+dD
the change in Gibbs free energy, ∆G (see Topic B6) for the reaction at a temperature, T,
is given by
where Q is the reaction quotient,