Page 100 - Formation Damage during Improved Oil Recovery Fundamentals and Applications
P. 100
82 Thomas Russell et al.
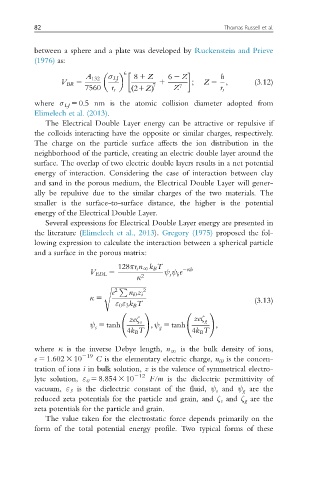
between a sphere and a plate was developed by Ruckenstein and Prieve
(1976) as:
6
A 132 σ LJ 8 1 Z 6 2 Z h
V BR 5 1 ; Z 5 ; (3.12)
7560 r s ð 21ZÞ 7 Z 7 r s
where σ LJ 5 0.5 nm is the atomic collision diameter adopted from
Elimelech et al. (2013).
The Electrical Double Layer energy can be attractive or repulsive if
the colloids interacting have the opposite or similar charges, respectively.
The charge on the particle surface affects the ion distribution in the
neighborhood of the particle, creating an electric double layer around the
surface. The overlap of two electric double layers results in a net potential
energy of interaction. Considering the case of interaction between clay
and sand in the porous medium, the Electrical Double Layer will gener-
ally be repulsive due to the similar charges of the two materials. The
smaller is the surface-to-surface distance, the higher is the potential
energy of the Electrical Double Layer.
Several expressions for Electrical Double Layer energy are presented in
the literature (Elimelech et al., 2013). Gregory (1975) proposed the fol-
lowing expression to calculate the interaction between a spherical particle
and a surface in the porous matrix:
128πr s n N k B T
V EDL 5 ψ ψ e 2κh
κ 2 s b
s ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2
2
P
e n i0 z i
κ 5 (3.13)
ε 0 ε 3 k B T
! !
zeζ zeζ g
ψ 5 tanh s ; ψ 5 tanh ;
g
s
4k B T 4k B T
where κ is the inverse Debye length, n N is the bulk density of ions,
e 5 1.602 3 10 219 C is the elementary electric charge, n i0 is the concen-
tration of ions i in bulk solution, z is the valence of symmetrical electro-
lyte solution, ε 0 5 8.854 3 10 212 F/m is the dielectric permittivity of
vacuum, ε 3 is the dielectric constant of the fluid, ψ s and ψ g are the
reduced zeta potentials for the particle and grain, and ζ s and ζ g are the
zeta potentials for the particle and grain.
The value taken for the electrostatic force depends primarily on the
form of the total potential energy profile. Two typical forms of these