Page 222 - Fundamentals of Air Pollution 3E
P. 222
II. Sampling Systems for Gaseous Pollutants 185
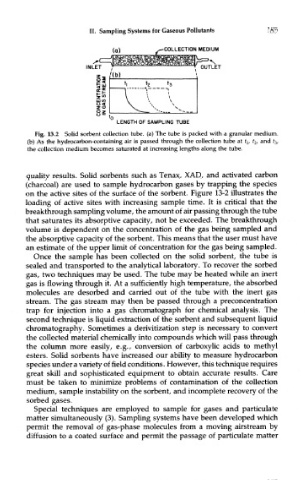
Fig. 13.2 Solid sorbent collection tube, (a) The tube is packed with a granular medium,
(b) As the hydrocarbon-containing air is passed through the collection tube at fj, t 2, and £ 3/
the collection medium becomes saturated at increasing lengths along the tube.
quality results. Solid sorbents such as Tenax, XAD, and activated carbon
(charcoal) are used to sample hydrocarbon gases by trapping the species
on the active sites of the surface of the sorbent. Figure 13-2 illustrates the
loading of active sites with increasing sample time. It is critical that the
breakthrough sampling volume, the amount of air passing through the tube
that saturates its absorptive capacity, not be exceeded. The breakthrough
volume is dependent on the concentration of the gas being sampled and
the absorptive capacity of the sorbent. This means that the user must have
an estimate of the upper limit of concentration for the gas being sampled.
Once the sample has been collected on the solid sorbent, the tube is
sealed and transported to the analytical laboratory. To recover the sorbed
gas, two techniques may be used. The tube may be heated while an inert
gas is flowing through it. At a sufficiently high temperature, the absorbed
molecules are desorbed and carried out of the tube with the inert gas
stream. The gas stream may then be passed through a preconcentration
trap for injection into a gas chromatograph for chemical analysis. The
second technique is liquid extraction of the sorbent and subsequent liquid
chromatography. Sometimes a derivitization step is necessary to convert
the collected material chemically into compounds which will pass through
the column more easily, e.g., conversion of carboxylic acids to methyl
esters. Solid sorbents have increased our ability to measure hydrocarbon
species under a variety of field conditions. However, this technique requires
great skill and sophisticated equipment to obtain accurate results. Care
must be taken to minimize problems of contamination of the collection
medium, sample instability on the sorbent, and incomplete recovery of the
sorbed gases.
Special techniques are employed to sample for gases and particulate
matter simultaneously (3). Sampling systems have been developed which
permit the removal of gas-phase molecules from a moving airstream by
diffusion to a coated surface and permit the passage of particulate matter