Page 109 - Fundamentals of Air Pollution
P. 109
II. Combustion 79
these relatively small quantities of materials emitted from the combustion
process. An automotive engineer, for example, was not overly concerned
about the 1% of carbon monoxide in the exhaust of the gasoline engine.
By getting this 1% to burn to carbon dioxide inside the combustion cham-
ber, the engineer could expect an increase in gasoline mileage of some-
thing less than one-half of 1%. This 1% of carbon monoxide, however, is
10,000 ppm by volume, and a number of such magnitude cannot be ignored
by an engineer dealing with air pollution problems.
Combustion is extremely complicated but is generally considered to be
a free radical chain reaction. Several reasons exist to support the free radical
mechanism. (1) Simple calculations of the heats of disassociation and forma-
tion for the molecules involved do not agree with the experimental values
obtained for heats of combustion. (2) A great variety of end products may
be found in the exhaust from a combustion reaction. Many complicated
organic molecules have been identified in the effluent from a system burn-
ing pure methane with pure oxygen. (3) Inhibitors, such as tetraethyl lead,
can greatly change the rate of reaction (3).
When visualizing a combustion process, it is useful to think of it in terms
of the three Ts: time, temperature, and turbulence. Time for combustion
to occur is necessary. A combustion process that is just initiated, and
suddenly has its reactants discharged to a chilled environment, will not go to
completion and will emit excessive pollutants. A high enough temperature
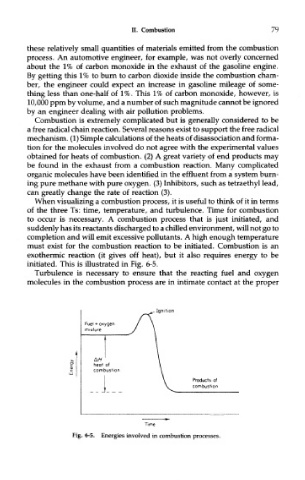
must exist for the combustion reaction to be initiated. Combustion is an
exothermic reaction (it gives off heat), but it also requires energy to be
initiated. This is illustrated in Fig. 6-5.
Turbulence is necessary to ensure that the reacting fuel and oxygen
molecules in the combustion process are in intimate contact at the proper
Fig. 6-5. Energies involved in combustion processes.