Page 214 - Fundamentals of Air Pollution
P. 214
180 13. Ambient Air Sampling
device measures the volume of air associated with the sampling system.
Examples of flow devices are mass flow meters, rotameters, and critical
orifices,
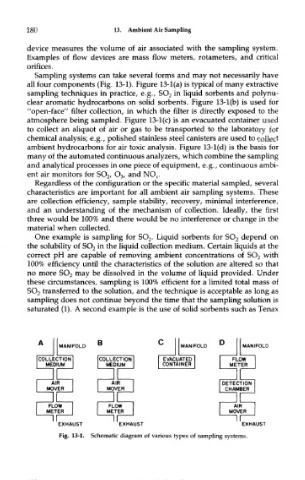
Sampling systems can take several forms and may not necessarily have
all four components (Fig. 13-1). Figure 13-l(a) is typical of many extractive
sampling techniques in practice, e.g., SO 2 in liquid sorbents and polynu-
clear aromatic hydrocarbons on solid sorbents. Figure 13-l(b) is used for
"open-face" filter collection, in which the filter is directly exposed to the
atmosphere being sampled. Figure 13-l(c) is an evacuated container used
to collect an aliquot of air or gas to be transported to the laboratory for
chemical analysis; e.g., polished stainless steel canisters are used to collect
ambient hydrocarbons for air toxic analysis. Figure 13-l(d) is the basis for
many of the automated continuous analyzers, which combine the sampling
and analytical processes in one piece of equipment, e.g., continuous ambi-
ent air monitors for SO 2, O 3, and NO X.
Regardless of the configuration or the specific material sampled, several
characteristics are important for all ambient air sampling systems. These
are collection efficiency, sample stability, recovery, minimal interference,
and an understanding of the mechanism of collection. Ideally, the first
three would be 100% and there would be no interference or change in the
material when collected.
One example is sampling for SO 2. Liquid sorbents for SO 2 depend on
the solubility of SO 2 in the liquid collection medium. Certain liquids at the
correct pH are capable of removing ambient concentrations of SO 2 with
100% efficiency until the characteristics of the solution are altered so that
no more SO 2 may be dissolved in the volume of liquid provided. Under
these circumstances, sampling is 100% efficient for a limited total mass of
SO 2 transferred to the solution, and the technique is acceptable as long as
sampling does not continue beyond the time that the sampling solution is
saturated (1). A second example is the use of solid sorbents such as Tenax
Fig. 13-1. Schematic diagram of various types of sampling systems.