Page 110 - Fundamentals of Computational Geoscience Numerical Methods and Algorithms
P. 110
98 5 Simulating Chemical Dissolution Front Instabilities in Fluid-Saturated Porous Rocks
(1992) state that “The Carman-Kozeny law is widely used since it seems to be the
best simple expression available.” For these reasons, the Carman-Kozeny law will
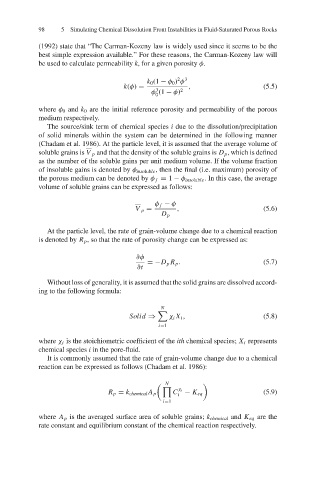
be used to calculate permeability k, for a given porosity φ.
2 3
k 0 (1 − φ 0 ) φ
k(φ) = 3 , (5.5)
φ (1 − φ) 2
0
where φ 0 and k 0 are the initial reference porosity and permeability of the porous
medium respectively.
The source/sink term of chemical species i due to the dissolution/precipitation
of solid minerals within the system can be determined in the following manner
(Chadam et al. 1986). At the particle level, it is assumed that the average volume of
soluble grains is V p and that the density of the soluble grains is D p , which is defined
as the number of the soluble gains per unit medium volume. If the volume fraction
of insoluble gains is denoted by φ insoluble , then the final (i.e. maximum) porosity of
the porous medium can be denoted by φ f = 1 − φ insoluble . In this case, the average
volume of soluble grains can be expressed as follows:
φ f − φ
V p = , (5.6)
D p
At the particle level, the rate of grain-volume change due to a chemical reaction
is denoted by R p , so that the rate of porosity change can be expressed as:
∂φ
=−D p R p . (5.7)
∂t
Without loss of generality, it is assumed that the solid grains are dissolved accord-
ing to the following formula:
N
Solid ⇒ χ i X i , (5.8)
i=1
where χ i is the stoichiometric coefficient of the ith chemical species; X i represents
chemical species i in the pore-fluid.
It is commonly assumed that the rate of grain-volume change due to a chemical
reaction can be expressed as follows (Chadam et al. 1986):
N
#
C χ i (5.9)
R p = k chemical A p − K eq
i
i=1
where A p is the averaged surface area of soluble grains; k chemical and K eq are the
rate constant and equilibrium constant of the chemical reaction respectively.