Page 50 - Fundamentals of Physical Volcanology
P. 50
9780632054435_4_002.qxd 12/10/2007 12:18PM Page 27
MAGMA GENERATION AND SEGREGATION 27
Before After occupy a smaller volume. They could in theory do
30 mm
this by moving away from the site of the melting,
but the viscosity of the solid crystal network is so
high that it cannot deform fast enough. The only
alternate is for the crystals surrounding a new melt
pocket to be compressed into a smaller volume.
To compress a solid, however, pressure must be
applied to it, and so as soon as any melt forms it
compresses all of the mineral grains in contact with
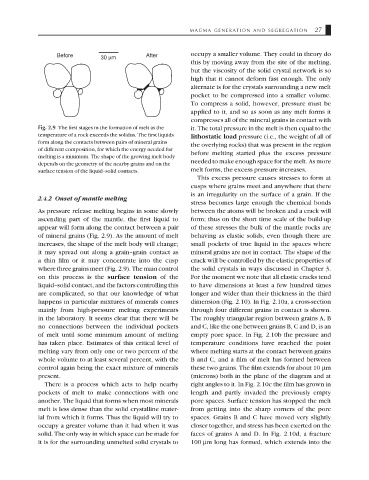
Fig. 2.9 The first stages in the formation of melt as the it. The total pressure in the melt is then equal to the
temperature of a rock exceeds the solidus. The first liquids lithostatic load pressure (i.e., the weight of all of
form along the contacts between pairs of mineral grains
the overlying rocks) that was present in the region
of different composition, for which the energy needed for
before melting started plus the excess pressure
melting is a minimum. The shape of the growing melt body
needed to make enough space for the melt. As more
depends on the geometry of the nearby grains and on the
surface tension of the liquid–solid contacts. melt forms, the excess pressure increases.
This excess pressure causes stresses to form at
cusps where grains meet and anywhere that there
is an irregularity on the surface of a grain. If the
2.4.2 Onset of mantle melting
stress becomes large enough the chemical bonds
As pressure release melting begins in some slowly between the atoms will be broken and a crack will
ascending part of the mantle, the first liquid to form; thus on the short time scale of the build-up
appear will form along the contact between a pair of these stresses the bulk of the mantle rocks are
of mineral grains (Fig. 2.9). As the amount of melt behaving as elastic solids, even though there are
increases, the shape of the melt body will change; small pockets of true liquid in the spaces where
it may spread out along a grain–grain contact as mineral grains are not in contact. The shape of the
a thin film or it may concentrate into the cusp crack will be controlled by the elastic properties of
where three grains meet (Fig. 2.9). The main control the solid crystals in ways discussed in Chapter 3.
on this process is the surface tension of the For the moment we note that all elastic cracks tend
liquid–solid contact, and the factors controlling this to have dimensions at least a few hundred times
are complicated, so that our knowledge of what longer and wider than their thickness in the third
happens in particular mixtures of minerals comes dimension (Fig. 2.10). In Fig. 2.10a, a cross-section
mainly from high-pressure melting experiments through four different grains in contact is shown.
in the laboratory. It seems clear that there will be The roughly triangular region between grains A, B
no connections between the individual pockets and C, like the one between grains B, C and D, is an
of melt until some minimum amount of melting empty pore space. In Fig. 2.10b the pressure and
has taken place. Estimates of this critical level of temperature conditions have reached the point
melting vary from only one or two percent of the where melting starts at the contact between grains
whole volume to at least several percent, with the B and C, and a film of melt has formed between
control again being the exact mixture of minerals these two grains. The film extends for about 10 µm
present. (microns) both in the plane of the diagram and at
There is a process which acts to help nearby right angles to it. In Fig. 2.10c the film has grown in
pockets of melt to make connections with one length and partly invaded the previously empty
another. The liquid that forms when most minerals pore spaces. Surface tension has stopped the melt
melt is less dense than the solid crystalline mater- from getting into the sharp corners of the pore
ial from which it forms. Thus the liquid will try to spaces. Grains B and C have moved very slightly
occupy a greater volume than it had when it was closer together, and stress has been exerted on the
solid. The only way in which space can be made for faces of grains A and D. In Fig. 2.10d, a fracture
it is for the surrounding unmelted solid crystals to 100 µm long has formed, which extends into the