Page 89 - Gas Purification 5E
P. 89
Alkanolamines for Hydrogen Surfide and Carbon Dioxide Removal 79
(text continued from page 64)
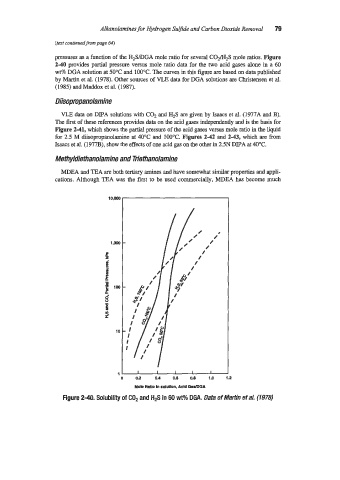
pressures as a function of the H2SDGA mole ratio for several C02/H2S mole ratios. Figure
2-40 provides partial pressure versus mole ratio data for the two acid gases alone in a 60
wt% DGA solution at 50°C and 100°C. The curves in this figure are based on data published
by Martin et al. (1978). Other sources of VLE data for DGA solutions are Christensen et al.
(1985) and Maddox et al. (1987).
Diisopro panolamine
VLE data on DIPA solutions with C02 and H2S are given by Isaacs et al. (1 977A and B).
The first of these references provides data on the acid gases independently and is the basis for
Figure 2-41, which shows the partial pressure of the acid gases versus mole ratio in the liquid
for 2.5 M diisopropanolamine at 40°C and 100°C. Figures 2-42 and 2-43, which are from
Isaacs et al. (1977B), show the effects of one acid gas on the other in 2.5N DIPA at 40°C.
Methyldiethanolamine and Triethanolamine
MDEA and TEA are both tertiary amines and have somewhat similar properties and appli-
cations. Although TEA was the first to be used commercially, MDEA has become much
0 0.2 0.4 0.6 0.6 1.0 1.2
Mole Ratlo in rolutlon, Acld O../DGA
Figure 2-40. Solubility of COP and H2S in 60 wt% DGA. Data of Martin eta/- (1978)