Page 102 - Gas Adsorption Equilibria
P. 102
88 Chapter 2
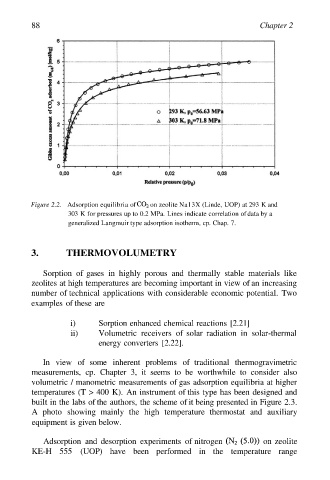
Figure 2.2. Adsorption equilibria of on zeolite Na13X (Linde, UOP) at 293 K and
303 K for pressures up to 0.2 MPa. Lines indicate correlation of data by a
generalized Langmuir type adsorption isotherm, cp. Chap. 7.
3. THERMOVOLUMETRY
Sorption of gases in highly porous and thermally stable materials like
zeolites at high temperatures are becoming important in view of an increasing
number of technical applications with considerable economic potential. Two
examples of these are
i) Sorption enhanced chemical reactions [2.21]
ii) Volumetric receivers of solar radiation in solar-thermal
energy converters [2.22].
In view of some inherent problems of traditional thermogravimetric
measurements, cp. Chapter 3, it seems to be worthwhile to consider also
volumetric / manometric measurements of gas adsorption equilibria at higher
temperatures (T > 400 K). An instrument of this type has been designed and
built in the labs of the authors, the scheme of it being presented in Figure 2.3.
A photo showing mainly the high temperature thermostat and auxiliary
equipment is given below.
Adsorption and desorption experiments of nitrogen on zeolite
KE-H 555 (UOP) have been performed in the temperature range