Page 205 - Gas Adsorption Equilibria
P. 205
4. Volumetric – Gravimetric Measurements 191
depending on the unknown masses of sorptive components
However, for low initial concentrations of the sorptive components, i. e.
i = 1,2, Z may be approximated by the real gas factor of the pure
carrier gas, i. e.
Coadsorption measurements of sorptive gases including inert components are
presently done at the author’s institution. Results will be published in a
forthcoming paper [4.7].
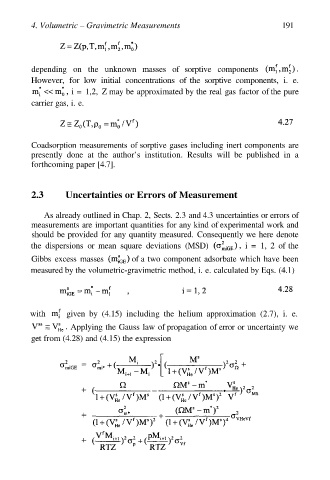
2.3 Uncertainties or Errors of Measurement
As already outlined in Chap. 2, Sects. 2.3 and 4.3 uncertainties or errors of
measurements are important quantities for any kind of experimental work and
should be provided for any quantity measured. Consequently we here denote
the dispersions or mean square deviations (MSD) i = 1, 2 of the
Gibbs excess masses of a two component adsorbate which have been
measured by the volumetric-gravimetric method, i. e. calculated by Eqs. (4.1)
with given by (4.15) including the helium approximation (2.7), i. e.
Applying the Gauss law of propagation of error or uncertainty we
get from (4.28) and (4.15) the expression