Page 96 - Gas Adsorption Equilibria
P. 96
82 Chapter 2
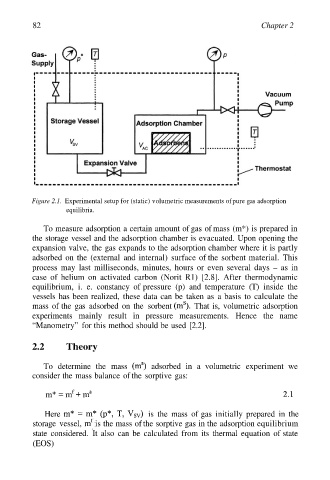
Figure 2.1. Experimental setup for (static) volumetric measurements of pure gas adsorption
equilibria.
To measure adsorption a certain amount of gas of mass (m*) is prepared in
the storage vessel and the adsorption chamber is evacuated. Upon opening the
expansion valve, the gas expands to the adsorption chamber where it is partly
adsorbed on the (external and internal) surface of the sorbent material. This
process may last milliseconds, minutes, hours or even several days – as in
case of helium on activated carbon (Norit R1) [2.8]. After thermodynamic
equilibrium, i. e. constancy of pressure (p) and temperature (T) inside the
vessels has been realized, these data can be taken as a basis to calculate the
mass of the gas adsorbed on the sorbent That is, volumetric adsorption
experiments mainly result in pressure measurements. Hence the name
“Manometry” for this method should be used [2.2].
2.2 Theory
To determine the mass adsorbed in a volumetric experiment we
consider the mass balance of the sorptive gas:
Here is the mass of gas initially prepared in the
storage vessel, is the mass of the sorptive gas in the adsorption equilibrium
state considered. It also can be calculated from its thermal equation of state
(EOS)