Page 22 - Geology of Carbonate Reservoirs
P. 22
DEFINITION OF CARBONATE RESERVOIRS 3
ment of atoms. The families are known by the crystal systems in which they form,
namely, the hexagonal, orthorhombic, and monoclinic crystallographic systems.
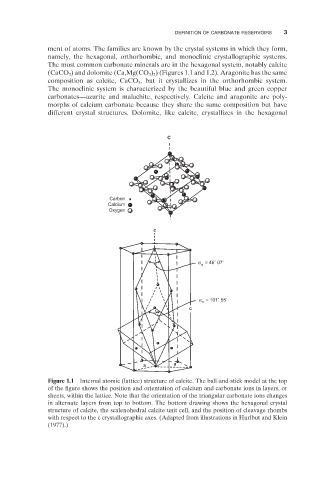
The most common carbonate minerals are in the hexagonal system, notably calcite
(CaCO 3 ) and dolomite (Ca,Mg(CO 3 ) 2 ) (Figures 1.1 and 1.2 ). Aragonite has the same
composition as calcite, CaCO 3 , but it crystallizes in the orthorhombic system.
The monoclinic system is characterized by the beautiful blue and green copper
carbonates — azurite and malachite, respectively. Calcite and aragonite are poly-
morphs of calcium carbonate because they share the same composition but have
different crystal structures. Dolomite, like calcite, crystallizes in the hexagonal
C
Carbon
Calcium
Oxygen
c
α = 46˚ 07’
R
α = 101˚ 55’
R
c
a a
Figure 1.1 Internal atomic (lattice) structure of calcite. The ball - and - stick model at the top
of the figure shows the position and orientation of calcium and carbonate ions in layers, or
sheets, within the lattice. Note that the orientation of the triangular carbonate ions changes
in alternate layers from top to bottom. The bottom drawing shows the hexagonal crystal
structure of calcite, the scalenohedral calcite unit cell, and the position of cleavage rhombs
with respect to the c crystallographic axes. (Adapted from illustrations in Hurlbut and Klein
(1977) .)