Page 101 - Handbook of Thermal Analysis of Construction Materials
P. 101
Section 4.0 - Synthesis of Cement Phases 85
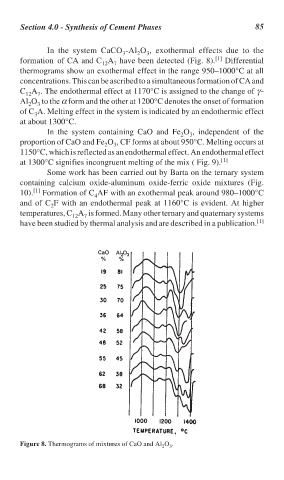
In the system CaCO -Al O , exothermal effects due to the
3
2
3
[1]
formation of CA and C A have been detected (Fig. 8). Differential
7
12
thermograms show an exothermal effect in the range 950–1000°C at all
concentrations. This can be ascribed to a simultaneous formation of CA and
C A . The endothermal effect at 1170°C is assigned to the change of γ-
12
7
Al O to the α form and the other at 1200°C denotes the onset of formation
2 3
of C A. Melting effect in the system is indicated by an endothermic effect
3
at about 1300°C.
In the system containing CaO and Fe O , independent of the
2
3
proportion of CaO and Fe O , CF forms at about 950°C. Melting occurs at
3
2
1150°C, which is reflected as an endothermal effect. An endothermal effect
at 1300°C signifies incongruent melting of the mix ( Fig. 9). [1]
Some work has been carried out by Barta on the ternary system
containing calcium oxide-aluminum oxide-ferric oxide mixtures (Fig.
[1]
10). Formation of C AF with an exothermal peak around 980–1000°C
4
and of C F with an endothermal peak at 1160°C is evident. At higher
2
temperatures, C A is formed. Many other ternary and quaternary systems
7
12
have been studied by thermal analysis and are described in a publication. [1]
Figure 8. Thermograms of mixtures of CaO and Al O .
2
3