Page 126 - Handbook of Thermal Analysis of Construction Materials
P. 126
110 Chapter 3 - Formation and Hydration
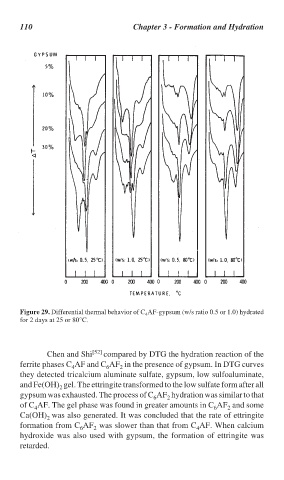
Figure 29. Differential thermal behavior of C AF-gypsum (w/s ratio 0.5 or 1.0) hydrated
4
for 2 days at 25 or 80°C.
Chen and Shi [52] compared by DTG the hydration reaction of the
ferrite phases C AF and C AF in the presence of gypsum. In DTG curves
4
6
2
they detected tricalcium aluminate sulfate, gypsum, low sulfoaluminate,
and Fe(OH) gel. The ettringite transformed to the low sulfate form after all
2
gypsum was exhausted. The process of C AF hydration was similar to that
6 2
of C AF. The gel phase was found in greater amounts in C AF and some
2
6
4
Ca(OH) was also generated. It was concluded that the rate of ettringite
2
formation from C AF was slower than that from C AF. When calcium
4
6
2
hydroxide was also used with gypsum, the formation of ettringite was
retarded.