Page 222 - Handbook of Thermal Analysis of Construction Materials
P. 222
Section 3.0 - Non-Chloride Accelerators 205
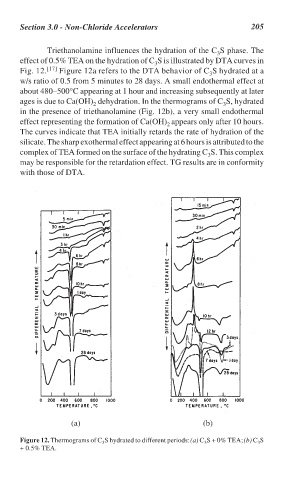
Triethanolamine influences the hydration of the C S phase. The
3
effect of 0.5% TEA on the hydration of C S is illustrated by DTA curves in
3
Fig. 12. [17] Figure 12a refers to the DTA behavior of C S hydrated at a
3
w/s ratio of 0.5 from 5 minutes to 28 days. A small endothermal effect at
about 480–500°C appearing at 1 hour and increasing subsequently at later
ages is due to Ca(OH) dehydration. In the thermograms of C S, hydrated
2 3
in the presence of triethanolamine (Fig. 12b), a very small endothermal
effect representing the formation of Ca(OH) appears only after 10 hours.
2
The curves indicate that TEA initially retards the rate of hydration of the
silicate. The sharp exothermal effect appearing at 6 hours is attributed to the
complex of TEA formed on the surface of the hydrating C S. This complex
3
may be responsible for the retardation effect. TG results are in conformity
with those of DTA.
(a) (b)
Figure 12. Thermograms of C S hydrated to different periods: (a) C S + 0% TEA; (b) C S
3
3
3
+ 0.5% TEA.