Page 233 - Handbook of Thermal Analysis of Construction Materials
P. 233
216 Chapter 5 - Accelerating Admixtures
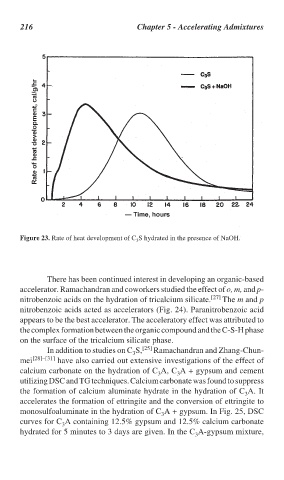
Figure 23. Rate of heat development of C S hydrated in the presence of NaOH.
3
There has been continued interest in developing an organic-based
accelerator. Ramachandran and coworkers studied the effect of o, m, and p-
nitrobenzoic acids on the hydration of tricalcium silicate. [27] The m and p
nitrobenzoic acids acted as accelerators (Fig. 24). Paranitrobenzoic acid
appears to be the best accelerator. The acceleratory effect was attributed to
the complex formation between the organic compound and the C-S-H phase
on the surface of the tricalcium silicate phase.
In addition to studies on C S, [25] Ramachandran and Zhang-Chun-
3
mei [28]–[31] have also carried out extensive investigations of the effect of
calcium carbonate on the hydration of C A, C A + gypsum and cement
3 3
utilizing DSC and TG techniques. Calcium carbonate was found to suppress
the formation of calcium aluminate hydrate in the hydration of C A. It
3
accelerates the formation of ettringite and the conversion of ettringite to
monosulfoaluminate in the hydration of C A + gypsum. In Fig. 25, DSC
3
curves for C A containing 12.5% gypsum and 12.5% calcium carbonate
3
hydrated for 5 minutes to 3 days are given. In the C A-gypsum mixture,
3