Page 25 - Handbook of Thermal Analysis of Construction Materials
P. 25
Section 2.0 - Classical Techniques 9
since they generate constant heat (i.e., power), which is independent of
temperature. Some of these materials, however, are not suitable at high
temperatures as they might diffuse through the sample holder. The most
often used radioactive material as a calibrant appears to be plutonium. [31]
The integration of a DSC (and a DTA) curve is directly propor-
tional to the enthalpy change, [32]
Eq. (1) Area = Km∆H
where K is the calibration coefficient, m the sample mass, and ∆H the heat
of transition. Unlike DTA, however, in DSC, K is temperature independent.
As is the case for DTA,* the term dH/dt for DSC is given by three measured
quantities, [32]
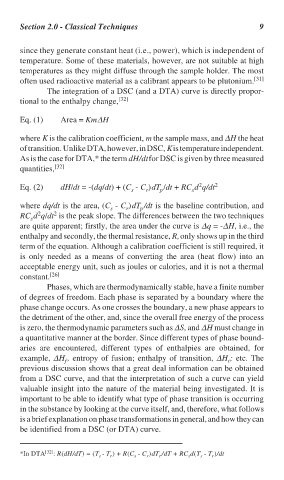
2
Eq. (2) dH/dt = -(dq/dt) + (C - C )dT /dt + RC d q/dt 2
r
p
s
s
where dq/dt is the area, (C - C )dT /dt is the baseline contribution, and
s
p
r
2
2
RC d q/dt is the peak slope. The differences between the two techniques
s
are quite apparent; firstly, the area under the curve is ∆q = -∆H, i.e., the
enthalpy and secondly, the thermal resistance, R, only shows up in the third
term of the equation. Although a calibration coefficient is still required, it
is only needed as a means of converting the area (heat flow) into an
acceptable energy unit, such as joules or calories, and it is not a thermal
constant. [26]
Phases, which are thermodynamically stable, have a finite number
of degrees of freedom. Each phase is separated by a boundary where the
phase change occurs. As one crosses the boundary, a new phase appears to
the detriment of the other, and, since the overall free energy of the process
is zero, the thermodynamic parameters such as ∆S, and ∆H must change in
a quantitative manner at the border. Since different types of phase bound-
aries are encountered, different types of enthalpies are obtained, for
example, ∆H , entropy of fusion; enthalpy of transition, ∆H ; etc. The
f t
previous discussion shows that a great deal information can be obtained
from a DSC curve, and that the interpretation of such a curve can yield
valuable insight into the nature of the material being investigated. It is
important to be able to identify what type of phase transition is occurring
in the substance by looking at the curve itself, and, therefore, what follows
is a brief explanation on phase transformations in general, and how they can
be identified from a DSC (or DTA) curve.
*In DTA [32] : R(dH/dT) = (T - T ) + R(C - C )dT /dT + RC d(T - T )/dt
s
s
r
r
s
s
r
r