Page 362 - Handbook of Thermal Analysis of Construction Materials
P. 362
Section 9.0 - Miscellaneous Additives 339
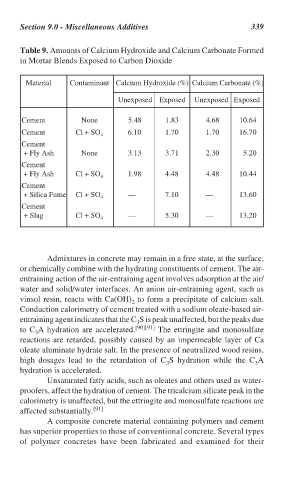
Table 9. Amounts of Calcium Hydroxide and Calcium Carbonate Formed
in Mortar Blends Exposed to Carbon Dioxide
Material Contaminant Calcium Hydroxide (%) Calcium Carbonate (%)
Unexposed Exposed Unexposed Exposed
Cement None 5.48 1.83 4.68 10.64
Cement Cl + SO 6.10 1.70 1.70 16.70
4
Cement
+ Fly Ash None 3.13 3.71 2.30 5.20
Cement
+ Fly Ash Cl + SO 4 1.98 4.48 4.48 10.44
Cement
+ Silica Fume Cl + SO — 7.10 — 13.60
4
Cement
+ Slag Cl + SO 4 — 5.30 — 13.20
Admixtures in concrete may remain in a free state, at the surface,
or chemically combine with the hydrating constituents of cement. The air-
entraining action of the air-entraining agent involves adsorption at the air/
water and solid/water interfaces. An anion air-entraining agent, such as
vinsol resin, reacts with Ca(OH) to form a precipitate of calcium salt.
2
Conduction calorimetry of cement treated with a sodium oleate-based air-
entraining agent indicates that the C S is peak unaffected, but the peaks due
3
to C A hydration are accelerated. [90][91] The ettringite and monosulfate
3
reactions are retarded, possibly caused by an impermeable layer of Ca
oleate aluminate hydrate salt. In the presence of neutralized wood resins,
high dosages lead to the retardation of C S hydration while the C A
3 3
hydration is accelerated.
Unsaturated fatty acids, such as oleates and others used as water-
proofers, affect the hydration of cement. The tricalcium silicate peak in the
calorimetry is unaffected, but the ettringite and monosulfate reactions are
affected substantially. [91]
A composite concrete material containing polymers and cement
has superior properties to those of conventional concrete. Several types
of polymer concretes have been fabricated and examined for their