Page 43 - Handbook of Thermal Analysis of Construction Materials
P. 43
Section 3.0 - Modern Techniques 27
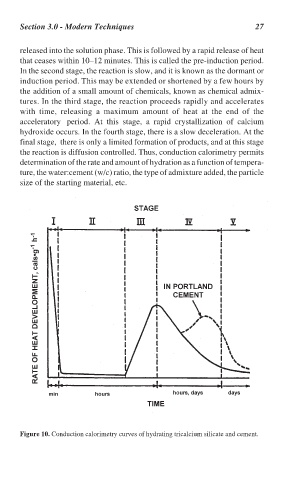
released into the solution phase. This is followed by a rapid release of heat
that ceases within 10–12 minutes. This is called the pre-induction period.
In the second stage, the reaction is slow, and it is known as the dormant or
induction period. This may be extended or shortened by a few hours by
the addition of a small amount of chemicals, known as chemical admix-
tures. In the third stage, the reaction proceeds rapidly and accelerates
with time, releasing a maximum amount of heat at the end of the
acceleratory period. At this stage, a rapid crystallization of calcium
hydroxide occurs. In the fourth stage, there is a slow deceleration. At the
final stage, there is only a limited formation of products, and at this stage
the reaction is diffusion controlled. Thus, conduction calorimetry permits
determination of the rate and amount of hydration as a function of tempera-
ture, the water:cement (w/c) ratio, the type of admixture added, the particle
size of the starting material, etc.
Figure 10. Conduction calorimetry curves of hydrating tricalcium silicate and cement.