Page 435 - Handbook of Thermal Analysis of Construction Materials
P. 435
Section 2.0 - Calcium Aluminate Cements 411
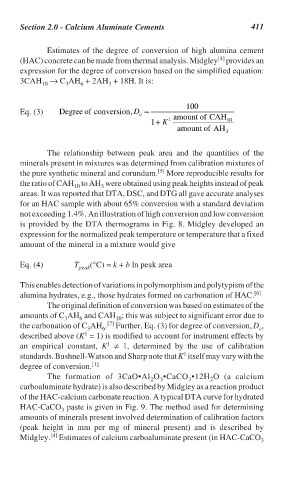
Estimates of the degree of conversion of high alumina cement
[4]
(HAC) concrete can be made from thermal analysis. Midgley provides an
expression for the degree of conversion based on the simplified equation:
3CAH → C AH + 2AH + 18H. It is:
10 3 6 3
Eq. (3) Degree of conversion ,D = 100
c 1 amount of CAH
+
1 K 10
amount of AH
3
The relationship between peak area and the quantities of the
minerals present in mixtures was determined from calibration mixtures of
[5]
the pure synthetic mineral and corundum. More reproducible results for
the ratio of CAH to AH were obtained using peak heights instead of peak
10 3
areas. It was reported that DTA, DSC, and DTG all gave accurate analyses
for an HAC sample with about 65% conversion with a standard deviation
not exceeding 1.4%. An illustration of high conversion and low conversion
is provided by the DTA thermograms in Fig. 8. Midgley developed an
expression for the normalized peak temperature or temperature that a fixed
amount of the mineral in a mixture would give
Eq. (4) T (°C) = k + b ln peak area
peak
This enables detection of variations in polymorphism and polytypism of the
alumina hydrates, e.g., those hydrates formed on carbonation of HAC. [6]
The original definition of conversion was based on estimates of the
amounts of C AH and CAH ; this was subject to significant error due to
3
6
10
[7]
the carbonation of C AH . Further, Eq. (3) for degree of conversion, D ,
3 6 c
1
described above (K = 1) is modified to account for instrument effects by
1
an empirical constant, K ≠ 1, determined by the use of calibration
1
standards. Bushnell-Watson and Sharp note that K itself may vary with the
degree of conversion. [1]
The formation of 3CaO•Al O •CaCO •12H O (a calcium
2
3
3
2
carboaluminate hydrate) is also described by Midgley as a reaction product
of the HAC-calcium carbonate reaction. A typical DTA curve for hydrated
HAC-CaCO paste is given in Fig. 9. The method used for determining
3
amounts of minerals present involved determination of calibration factors
(peak height in mm per mg of mineral present) and is described by
[4]
Midgley. Estimates of calcium carboaluminate present (in HAC-CaCO 3