Page 581 - Handbook of Thermal Analysis of Construction Materials
P. 581
Section 2.0 - Adhesives and Sealants 551
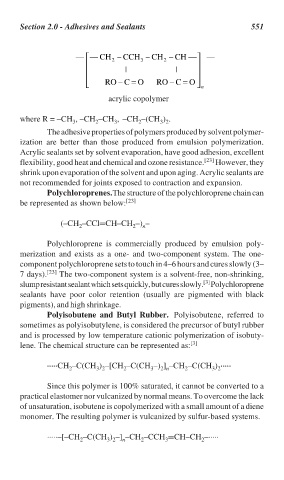
— — CH 2 – CCH 3 – CH 2 – CH — —
| |
RO – C = O RO – C = O n
acrylic copolymer
where R = –CH , –CH –CH , –CH –(CH ) .
2
3 2
3
3
2
The adhesive properties of polymers produced by solvent polymer-
ization are better than those produced from emulsion polymerization.
Acrylic sealants set by solvent evaporation, have good adhesion, excellent
flexibility, good heat and chemical and ozone resistance. [23] However, they
shrink upon evaporation of the solvent and upon aging. Acrylic sealants are
not recommended for joints exposed to contraction and expansion.
Polychloroprenes. The structure of the polychloroprene chain can
be represented as shown below: [23]
(–CH –CCl=CH–CH –) –
n
2
2
Polychloroprene is commercially produced by emulsion poly-
merization and exists as a one- and two-component system. The one-
component polychloroprene sets to touch in 4–6 hours and cures slowly (3–
7 days). [23] The two-component system is a solvent-free, non-shrinking,
[3]
slump resistant sealant which sets quickly, but cures slowly. Polychloroprene
sealants have poor color retention (usually are pigmented with black
pigments), and high shrinkage.
Polyisobutene and Butyl Rubber. Polyisobutene, referred to
sometimes as polyisobutylene, is considered the precursor of butyl rubber
and is processed by low temperature cationic polymerization of isobuty-
lene. The chemical structure can be represented as: [3]
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅ 2 3 2 2 3 2 n 2 3 2 ⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅
⋅⋅⋅⋅⋅CH –C(CH ) –[CH –C(CH –) ] –CH –C(CH ) ⋅⋅⋅⋅⋅
Since this polymer is 100% saturated, it cannot be converted to a
practical elastomer nor vulcanized by normal means. To overcome the lack
of unsaturation, isobutene is copolymerized with a small amount of a diene
monomer. The resulting polymer is vulcanized by sulfur-based systems.
⋅⋅⋅⋅⋅–[–CH –C(CH ) –] –CH –CCH =CH–CH –⋅⋅⋅⋅⋅
3 2
2
2
2
3
n