Page 106 - Geology and Geochemistry of Oil and Gas
P. 106
78 WATER
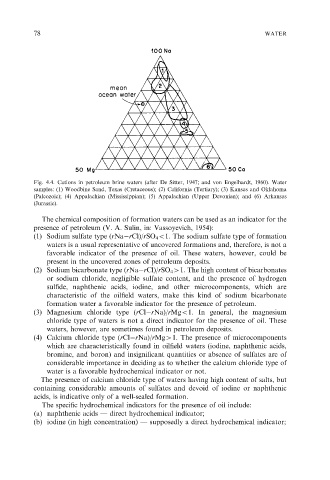
Fig. 4.4. Cations in petroleum brine waters (after De Sitter, 1947; and von Engelhardt, 1960). Water
samples: (1) Woodbine Sand, Texas (Cretaceous); (2) California (Tertiary); (3) Kansas and Oklahoma
(Paleozoic); (4) Appalachian (Mississippian); (5) Appalachian (Upper Devonian); and (6) Arkansas
(Jurassic).
The chemical composition of formation waters can be used as an indicator for the
presence of petroleum (V. A. Sulin, in: Vassoyevich, 1954):
(1) Sodium sulfate type (rNa rCl)/rSO 4 o1. The sodium sulfate type of formation
waters is a usual representative of uncovered formations and, therefore, is not a
favorable indicator of the presence of oil. These waters, however, could be
present in the uncovered zones of petroleum deposits.
(2) Sodium bicarbonate type (rNa rCl)/rSO 4 41. The high content of bicarbonates
or sodium chloride, negligible sulfate content, and the presence of hydrogen
sulfide, naphthenic acids, iodine, and other microcomponents, which are
characteristic of the oilfield waters, make this kind of sodium bicarbonate
formation water a favorable indicator for the presence of petroleum.
(3) Magnesium chloride type (rCl rNa)/rMgo1. In general, the magnesium
chloride type of waters is not a direct indicator for the presence of oil. These
waters, however, are sometimes found in petroleum deposits.
(4) Calcium chloride type (rCl rNa)/rMg41. The presence of microcomponents
which are characteristically found in oilfield waters (iodine, naphthenic acids,
bromine, and boron) and insignificant quantities or absence of sulfates are of
considerable importance in deciding as to whether the calcium chloride type of
water is a favorable hydrochemical indicator or not.
The presence of calcium chloride type of waters having high content of salts, but
containing considerable amounts of sulfates and devoid of iodine or naphthenic
acids, is indicative only of a well-sealed formation.
The specific hydrochemical indicators for the presence of oil include:
(a) naphthenic acids — direct hydrochemical indicator;
(b) iodine (in high concentration) — supposedly a direct hydrochemical indicator;