Page 132 - Geology and Geochemistry of Oil and Gas
P. 132
ISOTOPE COMPOSITION OF NATURAL GASES 103
The H 2 S content in the hydrocarbon gases ranges between 0 and a few percentage
points. At a significant depth (deeper than 4,500 m) and in a predominantly car-
bonate section it may be higher than 20% (Astrakhan Field in Russia, Tengiz Field
in Kazakhstan, and Emory Field in the U.S.).
Most common of the inert gases is helium. Its average concentration is 0.01–0.2%
and rises to 10% on the ancient platforms. The argon content ranges from 0.001 to
0.1%, sometimes up to 1–2% in helium-rich gases.
6.2. ISOTOPE COMPOSITION OF NATURAL GASES
6.2.1. Carbon
The carbon isotope composition in hydrocarbon gases varies from 40% to 50%
(and higher).
Gases form biochemically in near-surface environment from plant and animal
remains. Such gases have a high methane content (90% and higher). This methane is
12
enriched in the light carbon isotope ( C) compared with the carbon of organic
matter (up to 50% and higher).
The carbon of methane in natural gases is isotopically heavier than the carbon of
methane of biochemical origin, but lighter (by 10–20%) than the carbon of crude
13
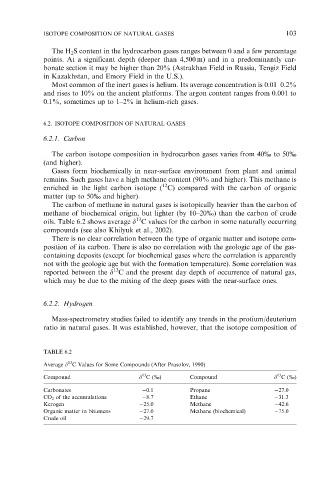
oils. Table 6.2 shows average d C values for the carbon in some naturally occurring
compounds (see also Khilyuk et al., 2002).
There is no clear correlation between the type of organic matter and isotope com-
position of its carbon. There is also no correlation with the geologic age of the gas-
containing deposits (except for biochemical gases where the correlation is apparently
not with the geologic age but with the formation temperature). Some correlation was
13
reported between the d C and the present day depth of occurrence of natural gas,
which may be due to the mixing of the deep gases with the near-surface ones.
6.2.2. Hydrogen
Mass-spectrometry studies failed to identify any trends in the protium/deuterium
ratio in natural gases. It was established, however, that the isotope composition of
TABLE 6.2
13
Average d C Values for Some Compounds (After Prasolov, 1990)
13
13
Compound d C (%) Compound d C (%)
Carbonates 0.1 Propane 27.0
CO 2 of the accumulations 8.7 Ethane 31.3
Kerogen 25.0 Methane 42.6
Organic matter in bitumens 27.0 Methane (biochemical) 75.0
Crude oil 29.7