Page 47 - Geology and Geochemistry of Oil and Gas
P. 47
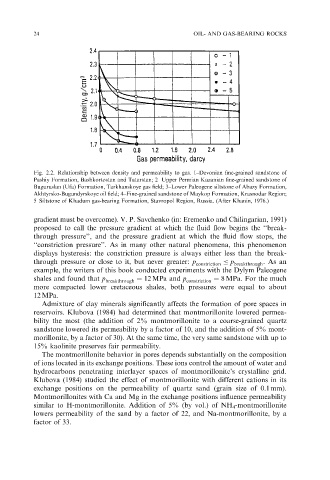
24 OIL- AND GAS-BEARING ROCKS
Fig. 2.2. Relationship between density and permeability to gas. 1–Devonian fine-grained sandstone of
Pashiy Formation, Bashkortostan and Tatarstan; 2– Upper Permian Kazanian fine-grained sandstone of
Buguruslan (Ufa) Formation, Tarkhanskoye gas field; 3–Lower Paleogene siltstone of Abazy Formation,
Akhtyrsko-Bugundyrskoye oil field; 4–Fine-grained sandstone of Maykop Formation, Krasnodar Region;
5–Siltstone of Khadum gas-bearing Formation, Stavropol Region, Russia. (After Khanin, 1976.)
gradient must be overcome). V. P. Savchenko (in: Eremenko and Chilingarian, 1991)
proposed to call the pressure gradient at which the fluid flow begins the ‘‘break-
through pressure’’, and the pressure gradient at which the fluid flow stops, the
‘‘constriction pressure’’. As in many other natural phenomena, this phenomenon
displays hysteresis: the constriction pressure is always either less than the break-
through pressure or close to it, but never greater: p constriction p breakthrough . As an
example, the writers of this book conducted experiments with the Dylym Paleogene
shales and found that p breakthrough ¼ 12 MPa and p constriction ¼ 8 MPa. For the much
more compacted lower cretaceous shales, both pressures were equal to about
12 MPa.
Admixture of clay minerals significantly affects the formation of pore spaces in
reservoirs. Klubova (1984) had determined that montmorillonite lowered permea-
bility the most (the addition of 2% montmorillonite to a coarse-grained quartz
sandstone lowered its permeability by a factor of 10, and the addition of 5% mont-
morillonite, by a factor of 30). At the same time, the very same sandstone with up to
15% kaolinite preserves fair permeability.
The montmorillonite behavior in pores depends substantially on the composition
of ions located in its exchange positions. These ions control the amount of water and
hydrocarbons penetrating interlayer spaces of montmorillonite’s crystalline grid.
Klubova (1984) studied the effect of montmorillonite with different cations in its
exchange positions on the permeability of quartz sand (grain size of 0.1 mm).
Montmorillonites with Ca and Mg in the exchange positions influence permeability
similar to H-montmorillonite. Addition of 5% (by vol.) of NH 4 -montmorillonite
lowers permeability of the sand by a factor of 22, and Na-montmorillonite, by a
factor of 33.