Page 71 - Geology and Geochemistry of Oil and Gas
P. 71
DEFORMATION OF ROCKS IN DEPTH 47
A lower pressure, as compared with the surrounding shales, forms within the
upper portion of the Zone III during subsidence. As mentioned before, the
montmorillonite-to-illite transformation is accompanied by the release of water and
shale loosening, after which shales compact further. Released water (that had been
previously chemically bonded) is chemically aggressive and dissolves various salts as
well as hydrocarbons in the surrounding rocks.
Pressure inversion occurs in Zone IV down the section. Reservoir pressure exceeds
the pore pressure in shales. Silicified reservoir rocks become fluid barriers, whereas
compacted argillaceous rocks experience fracturing and become reservoir rocks.
The described phenomena that occur at elevated subsurface pressures and
temperatures is significantly complicated and intensified because of some other
energy sources. These sources include tectonic stresses, seismic activity, changes in
the energy and magnetic fields, and exothermal reactions of mechanochemical
nature. Minskiy (1975, p. 128) pointed out that ‘‘Domains where reservoir rocks
have optimal properties are most favorable for the phase transitions of hydrocarbons
due to the decline of pore pressure in reservoir rocks. Salt concentration in formation
water changes, which,y, facilitates the release of hydrocarbons as a free phase and
the concentration of its emulsions.’’
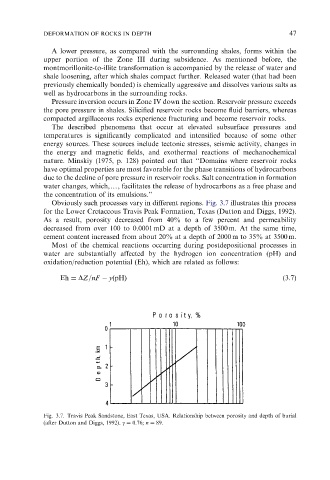
Obviously such processes vary in different regions. Fig. 3.7 illustrates this process
for the Lower Cretaceous Travis Peak Formation, Texas (Dutton and Diggs, 1992).
As a result, porosity decreased from 40% to a few percent and permeability
decreased from over 100 to 0.0001 mD at a depth of 3500 m. At the same time,
cement content increased from about 20% at a depth of 2000 m to 35% at 3500 m.
Most of the chemical reactions occurring during postdepositional processes in
water are substantially affected by the hydrogen ion concentration (pH) and
oxidation/reduction potential (Eh), which are related as follows:
Eh ¼ DZ=nF yðpHÞ (3.7)
Fig. 3.7. Travis Peak Sandstone, East Texas, USA. Relationship between porosity and depth of burial
(after Dutton and Diggs, 1992). g ¼ 0.76; n ¼ 89.