Page 165 - Handbook of Thermal Analysis of Construction Materials
P. 165
148 Chapter 4 - Introduction to Concrete Admixtures
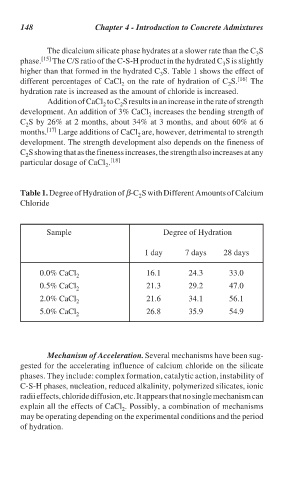
The dicalcium silicate phase hydrates at a slower rate than the C S
3
phase. [15] The C/S ratio of the C-S-H product in the hydrated C S is slightly
3
higher than that formed in the hydrated C S. Table 1 shows the effect of
3
different percentages of CaCl on the rate of hydration of C S. [16] The
2 2
hydration rate is increased as the amount of chloride is increased.
Addition of CaCl to C S results in an increase in the rate of strength
2 2
development. An addition of 3% CaCl increases the bending strength of
2
C S by 26% at 2 months, about 34% at 3 months, and about 60% at 6
2
months. [17] Large additions of CaCl are, however, detrimental to strength
2
development. The strength development also depends on the fineness of
C S showing that as the fineness increases, the strength also increases at any
2
particular dosage of CaCl . [18]
2
Table 1. Degree of Hydration of β-C S with Different Amounts of Calcium
2
Chloride
Sample Degree of Hydration
1 day 7 days 28 days
0.0% CaCl 16.1 24.3 33.0
2
0.5% CaCl 21.3 29.2 47.0
2
2.0% CaCl 21.6 34.1 56.1
2
5.0% CaCl 2 26.8 35.9 54.9
Mechanism of Acceleration. Several mechanisms have been sug-
gested for the accelerating influence of calcium chloride on the silicate
phases. They include: complex formation, catalytic action, instability of
C-S-H phases, nucleation, reduced alkalinity, polymerized silicates, ionic
radii effects, chloride diffusion, etc. It appears that no single mechanism can
explain all the effects of CaCl . Possibly, a combination of mechanisms
2
may be operating depending on the experimental conditions and the period
of hydration.