Page 212 - Handbook of Thermal Analysis of Construction Materials
P. 212
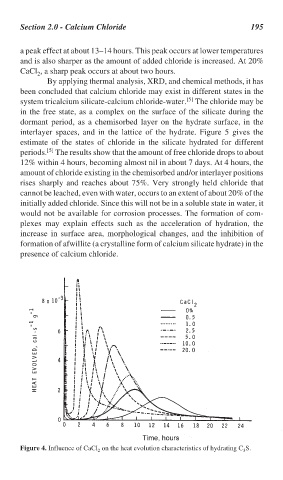
Section 2.0 - Calcium Chloride 195
a peak effect at about 13–14 hours. This peak occurs at lower temperatures
and is also sharper as the amount of added chloride is increased. At 20%
CaCl , a sharp peak occurs at about two hours.
2
By applying thermal analysis, XRD, and chemical methods, it has
been concluded that calcium chloride may exist in different states in the
[5]
system tricalcium silicate-calcium chloride-water. The chloride may be
in the free state, as a complex on the surface of the silicate during the
dormant period, as a chemisorbed layer on the hydrate surface, in the
interlayer spaces, and in the lattice of the hydrate. Figure 5 gives the
estimate of the states of chloride in the silicate hydrated for different
[5]
periods. The results show that the amount of free chloride drops to about
12% within 4 hours, becoming almost nil in about 7 days. At 4 hours, the
amount of chloride existing in the chemisorbed and/or interlayer positions
rises sharply and reaches about 75%. Very strongly held chloride that
cannot be leached, even with water, occurs to an extent of about 20% of the
initially added chloride. Since this will not be in a soluble state in water, it
would not be available for corrosion processes. The formation of com-
plexes may explain effects such as the acceleration of hydration, the
increase in surface area, morphological changes, and the inhibition of
formation of afwillite (a crystalline form of calcium silicate hydrate) in the
presence of calcium chloride.
Figure 4. Influence of CaCl on the heat evolution characteristics of hydrating C S.
2 3