Page 258 - Handbook of Thermal Analysis of Construction Materials
P. 258
Section 5.0 - Sugars 239
A complex results when a cement is hydrated in the presence of
salicylic acid. Such a product, when added to cement, slows down the
hydration. TG and other methods have been used to identify this
complex. [25][26]
5.0 SUGARS
The retarding action of sugars and their oxidation products on
concrete have been well documented. All saccharides with the exception of
trehalose strongly retard the set of cement. The five-membered rings,
sucrose and raffinose, are the best retarders. The reducing sugar glucose,
maltose, and lactose are moderate retarders. The strong retarding action of
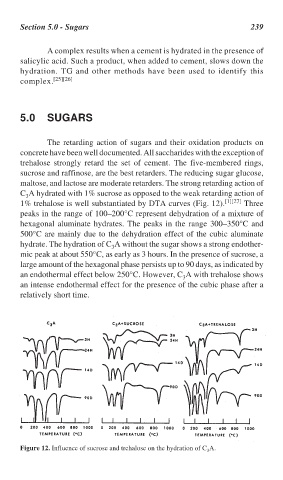
C A hydrated with 1% sucrose as opposed to the weak retarding action of
3
1% trehalose is well substantiated by DTA curves (Fig. 12). [1][27] Three
peaks in the range of 100–200°C represent dehydration of a mixture of
hexagonal aluminate hydrates. The peaks in the range 300–350°C and
500°C are mainly due to the dehydration effect of the cubic aluminate
hydrate. The hydration of C A without the sugar shows a strong endother-
3
mic peak at about 550°C, as early as 3 hours. In the presence of sucrose, a
large amount of the hexagonal phase persists up to 90 days, as indicated by
an endothermal effect below 250°C. However, C A with trehalose shows
3
an intense endothermal effect for the presence of the cubic phase after a
relatively short time.
Figure 12. Influence of sucrose and trehalose on the hydration of C A.
3