Page 342 - Handbook of Thermal Analysis of Construction Materials
P. 342
318 Chapter 8 - Supplementary Cementing Materials
The slag by itself is not hydraulic, but treating it with an activator
enables it to react with water. Conduction calorimetry and DTA techniques
are suited to evaluate the efficiency of activators for slags. Thermal
techniques have been applied to study the effectiveness of activators, such
as NaOH, Na CO , and Na SiO , [49] NaBr, NaI, Na SO , [50] etc., and water
2
2
4
3
2
3
glass. [51] The DTA of the slag-gypsum anhydrite mixture activated by small
amounts of sodium sulfate, calcium hydroxide, and ferrous sulfate is shown
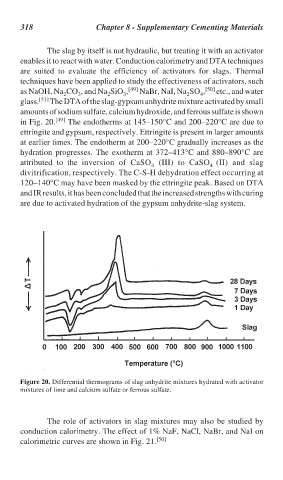
in Fig. 20. [49] The endotherms at 145–150°C and 200–220°C are due to
ettringite and gypsum, respectively. Ettringite is present in larger amounts
at earlier times. The endotherm at 200–220°C gradually increases as the
hydration progresses. The exotherm at 372–413°C and 880–890°C are
attributed to the inversion of CaSO (III) to CaSO (II) and slag
4 4
divitrification, respectively. The C-S-H dehydration effect occurring at
120–140°C may have been masked by the ettringite peak. Based on DTA
and IR results, it has been concluded that the increased strengths with curing
are due to activated hydration of the gypsum anhydrite-slag system.
Figure 20. Differential thermograms of slag anhydrite mixtures hydrated with activator
mixtures of lime and calcium sulfate or ferrous sulfate.
The role of activators in slag mixtures may also be studied by
conduction calorimetry. The effect of 1% NaF, NaCl, NaBr, and NaI on
calorimetric curves are shown in Fig. 21. [50]