Page 350 - Handbook of Thermal Analysis of Construction Materials
P. 350
326 Chapter 8 - Supplementary Cementing Materials
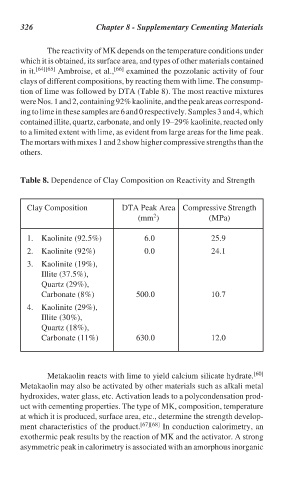
The reactivity of MK depends on the temperature conditions under
which it is obtained, its surface area, and types of other materials contained
in it. [64][65] Ambroise, et al., [66] examined the pozzolanic activity of four
clays of different compositions, by reacting them with lime. The consump-
tion of lime was followed by DTA (Table 8). The most reactive mixtures
were Nos. 1 and 2, containing 92% kaolinite, and the peak areas correspond-
ing to lime in these samples are 6 and 0 respectively. Samples 3 and 4, which
contained illite, quartz, carbonate, and only 19–29% kaolinite, reacted only
to a limited extent with lime, as evident from large areas for the lime peak.
The mortars with mixes 1 and 2 show higher compressive strengths than the
others.
Table 8. Dependence of Clay Composition on Reactivity and Strength
Clay Composition DTA Peak Area Compressive Strength
2
(mm ) (MPa)
1. Kaolinite (92.5%) 6.0 25.9
2. Kaolinite (92%) 0.0 24.1
3. Kaolinite (19%),
Illite (37.5%),
Quartz (29%),
Carbonate (8%) 500.0 10.7
4. Kaolinite (29%),
Illite (30%),
Quartz (18%),
Carbonate (11%) 630.0 12.0
Metakaolin reacts with lime to yield calcium silicate hydrate. [60]
Metakaolin may also be activated by other materials such as alkali metal
hydroxides, water glass, etc. Activation leads to a polycondensation prod-
uct with cementing properties. The type of MK, composition, temperature
at which it is produced, surface area, etc., determine the strength develop-
ment characteristics of the product. [67][68] In conduction calorimetry, an
exothermic peak results by the reaction of MK and the activator. A strong
asymmetric peak in calorimetry is associated with an amorphous inorganic