Page 442 - Handbook of Thermal Analysis of Construction Materials
P. 442
Section 2.0 - Calcium Aluminate Cements 417
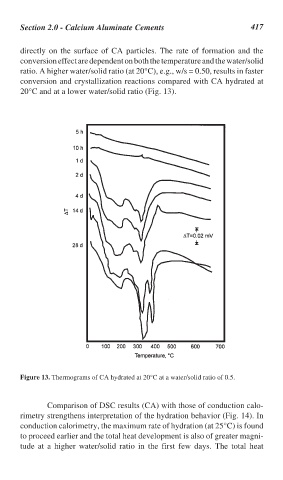
directly on the surface of CA particles. The rate of formation and the
conversion effect are dependent on both the temperature and the water/solid
ratio. A higher water/solid ratio (at 20°C), e.g., w/s = 0.50, results in faster
conversion and crystallization reactions compared with CA hydrated at
20°C and at a lower water/solid ratio (Fig. 13).
Figure 13. Thermograms of CA hydrated at 20°C at a water/solid ratio of 0.5.
Comparison of DSC results (CA) with those of conduction calo-
rimetry strengthens interpretation of the hydration behavior (Fig. 14). In
conduction calorimetry, the maximum rate of hydration (at 25°C) is found
to proceed earlier and the total heat development is also of greater magni-
tude at a higher water/solid ratio in the first few days. The total heat