Page 71 - Handbook of Thermal Analysis of Construction Materials
P. 71
Section 6.0 - Properties of Cement Paste 53
The above list is not exclusive because other forms have also been
reported.
6.3 Bond Formation
Cementitious materials, such as gypsum, portland cement, magne-
sium oxychloride, and alumina cement form porous bodies, and explana-
tions of their mechanical properties should take into account the nature of
the void spaces and the solid portion. If the solid part determines strength,
then several factors should be considered including the rate of dissolution
and solubility of the cement, the role of nuclei and their growth, chemical
and physical nature of the products, and energetics of the surface and
interfacial bonds.
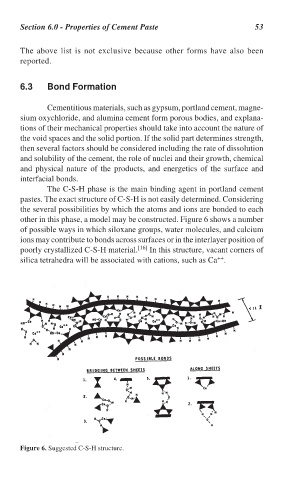
The C-S-H phase is the main binding agent in portland cement
pastes. The exact structure of C-S-H is not easily determined. Considering
the several possibilities by which the atoms and ions are bonded to each
other in this phase, a model may be constructed. Figure 6 shows a number
of possible ways in which siloxane groups, water molecules, and calcium
ions may contribute to bonds across surfaces or in the interlayer position of
poorly crystallized C-S-H material. [16] In this structure, vacant corners of
++
silica tetrahedra will be associated with cations, such as Ca .
Figure 6. Suggested C-S-H structure.