Page 265 - Handbook of Adhesives and Sealants
P. 265
Surfaces and Surface Preparation 233
the bond by providing a weak boundary layer before the adhesive is
applied. Corrosion can also occur after the joint is made and, thereby,
affect its permanence. Mechanical abrasion or solvent cleaning can
provide adhesive joints that are strong in dry conditions. However, this
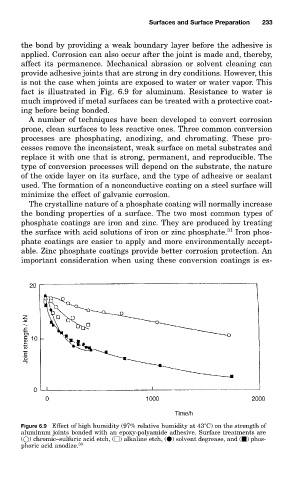
is not the case when joints are exposed to water or water vapor. This
fact is illustrated in Fig. 6.9 for aluminum. Resistance to water is
much improved if metal surfaces can be treated with a protective coat-
ing before being bonded.
A number of techniques have been developed to convert corrosion
prone, clean surfaces to less reactive ones. Three common conversion
processes are phosphating, anodizing, and chromating. These pro-
cesses remove the inconsistent, weak surface on metal substrates and
replace it with one that is strong, permanent, and reproducible. The
type of conversion processes will depend on the substrate, the nature
of the oxide layer on its surface, and the type of adhesive or sealant
used. The formation of a nonconductive coating on a steel surface will
minimize the effect of galvanic corrosion.
The crystalline nature of a phosphate coating will normally increase
the bonding properties of a surface. The two most common types of
phosphate coatings are iron and zinc. They are produced by treating
the surface with acid solutions of iron or zinc phosphate. 31 Iron phos-
phate coatings are easier to apply and more environmentally accept-
able. Zinc phosphate coatings provide better corrosion protection. An
important consideration when using these conversion coatings is es-
Figure 6.9 Effect of high humidity (97% relative humidity at 43 C) on the strength of
aluminum joints bonded with an epoxy-polyamide adhesive. Surface treatments are
( ) chromic–sulfuric acid etch, ( ) alkaline etch, ( ) solvent degrease, and ( ) phos-
phoric acid anodize. 30