Page 34 - Handbook of Adhesives and Sealants
P. 34
4 Chapter One
to bond weak substrates. Examples of non-structural adhesives are
pressure sensitive films, wood glue, elastomers, and sealants.
Sealants are generally chosen for their ability to fill gaps, resist
relative movement of the substrates, and exclude or contain another
material. They are generally lower in strength than adhesives, but
have better flexibility. Common sealants include urethanes, silicones,
and acrylic systems.
Both adhesives and sealants function primarily by the property of
adhesion. Adhesion is the attraction of two different substances re-
sulting from intermolecular forces between the substances. This is dis-
tinctly different from cohesion, which involves only the intermolecular
attractive forces within a single substance. The intermolecular forces
acting in both adhesion and cohesion are primarily van der Waals
forces which will be explained in the next chapter. To better under-
stand the difference between adhesion and cohesion, consider the
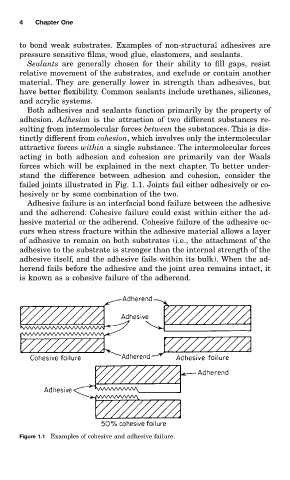
failed joints illustrated in Fig. 1.1. Joints fail either adhesively or co-
hesively or by some combination of the two.
Adhesive failure is an interfacial bond failure between the adhesive
and the adherend. Cohesive failure could exist within either the ad-
hesive material or the adherend. Cohesive failure of the adhesive oc-
curs when stress fracture within the adhesive material allows a layer
of adhesive to remain on both substrates (i.e., the attachment of the
adhesive to the substrate is stronger than the internal strength of the
adhesive itself, and the adhesive fails within its bulk). When the ad-
herend fails before the adhesive and the joint area remains intact, it
is known as a cohesive failure of the adherend.
Figure 1.1 Examples of cohesive and adhesive failure.