Page 407 - Handbook of Battery Materials
P. 407
13.2 Surface of Uncycled Lithium Foil 379
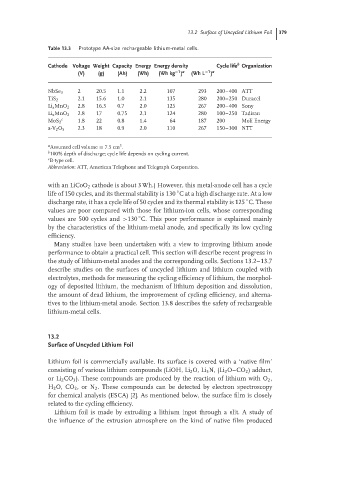
Table 13.3 Prototype AA-size rechargeable lithium-metal cells.
b
Cathode Voltage Weight Capacity Energy Energy density Cycle life Organization
−1 a
−1 a
(V) (g) (Ah) (Wh) (Wh kg ) (Wh L )
NbSe 3 2 20.5 1.1 2.2 107 293 200–400 ATT
TiS 2 2.1 15.6 1.0 2.1 135 280 200–250 Duracel
2.8 16.3 0.7 2.0 125 267 200–400 Sony
Li x MnO 2
Li x MnO 2 2.8 17 0.75 2.1 124 280 100–250 Tadiran
c
MoS 2 1.8 22 0.8 1.4 64 187 200 Moli Energy
a-V 2 O 5 2.3 18 0.9 2.0 110 267 150–300 NTT
a Assumed cell volume = 7.5cm .
3
b
100% depth of discharge; cycle life depends on cycling current.
c B-type cell.
Abbreviation: ATT, American Telephone and Telegraph Corporation.
with an LiCoO 2 cathode is about 3 Wh.) However, this metal-anode cell has a cycle
◦
life of 150 cycles, and its thermal stability is 130 C at a high discharge rate. At a low
◦
discharge rate, it has a cycle life of 50 cycles and its thermal stability is 125 C. These
values are poor compared with those for lithium-ion cells, whose corresponding
values are 500 cycles and >130 C. This poor performance is explained mainly
◦
by the characteristics of the lithium-metal anode, and specifically its low cycling
efficiency.
Many studies have been undertaken with a view to improving lithium anode
performance to obtain a practical cell. This section will describe recent progress in
the study of lithium-metal anodes and the corresponding cells. Sections 13.2–13.7
describe studies on the surfaces of uncycled lithium and lithium coupled with
electrolytes, methods for measuring the cycling efficiency of lithium, the morphol-
ogy of deposited lithium, the mechanism of lithium deposition and dissolution,
the amount of dead lithium, the improvement of cycling efficiency, and alterna-
tives to the lithium-metal anode. Section 13.8 describes the safety of rechargeable
lithium-metal cells.
13.2
Surface of Uncycled Lithium Foil
Lithium foil is commercially available. Its surface is covered with a ‘native film’
consisting of various lithium compounds (LiOH, Li 2 O, Li 3 N, (Li 2 O–CO 2 ) adduct,
or Li 2 CO 3 ). These compounds are produced by the reaction of lithium with O 2 ,
H 2 O, CO 2 ,orN 2 . These compounds can be detected by electron spectroscopy
for chemical analysis (ESCA) [2]. As mentioned below, the surface film is closely
related to the cycling efficiency.
Lithium foil is made by extruding a lithium ingot through a slit. A study of
the influence of the extrusion atmosphere on the kind of native film produced