Page 45 - Handbook of Electrical Engineering
P. 45
24 HANDBOOK OF ELECTRICAL ENGINEERING
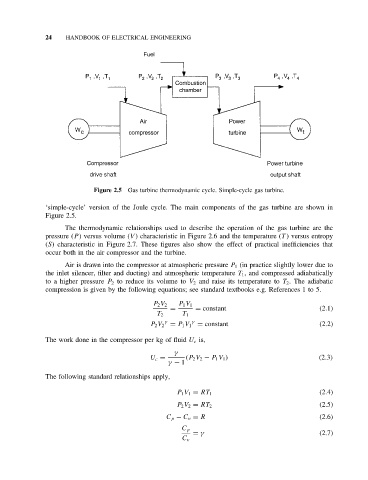
Figure 2.5 Gas turbine thermodynamic cycle. Simple-cycle gas turbine.
‘simple-cycle’ version of the Joule cycle. The main components of the gas turbine are shown in
Figure 2.5.
The thermodynamic relationships used to describe the operation of the gas turbine are the
pressure (P ) versus volume (V ) characteristic in Figure 2.6 and the temperature (T ) versus entropy
(S) characteristic in Figure 2.7. These figures also show the effect of practical inefficiencies that
occur both in the air compressor and the turbine.
Air is drawn into the compressor at atmospheric pressure P 1 (in practice slightly lower due to
the inlet silencer, filter and ducting) and atmospheric temperature T 1 , and compressed adiabatically
to a higher pressure P 2 to reduce its volume to V 2 and raise its temperature to T 2 . The adiabatic
compression is given by the following equations; see standard textbooks e.g. References 1 to 5.
P 2 V 2 P 1 V 1
= = constant (2.1)
T 2 T 1
γ
γ
P 2 V 2 = P 1 V 1 = constant (2.2)
The work done in the compressor per kg of fluid U c is,
γ
U c = (P 2 V 2 − P 1 V 1 ) (2.3)
γ − 1
The following standard relationships apply,
P 1 V 1 = RT 1 (2.4)
P 2 V 2 = RT 2 (2.5)
C p − C v = R (2.6)
C p
= γ (2.7)
C v