Page 129 - Handbook of Energy Engineering Calculations
P. 129
A gas turbine combustion chamber is well insulated so that heat losses to the
atmosphere are negligible. Octane, C H , is to be used as the fuel and 400
8 18
percent of the stoichiometric air quantity is to be supplied. The air first passes
through a regenerative heater and the air supply temperature at the
combustion chamber inlet is to be set so that the exit temperature of the
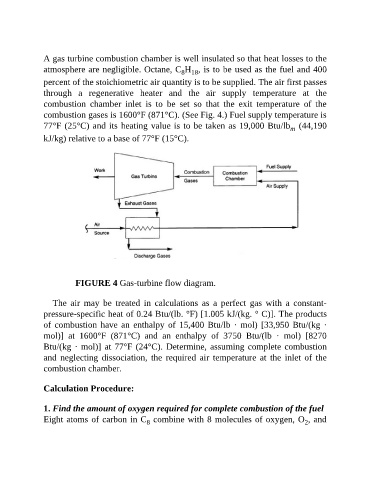
combustion gases is 1600°F (871°C). (See Fig. 4.) Fuel supply temperature is
77°F (25°C) and its heating value is to be taken as 19,000 Btu/lb (44,190
m
kJ/kg) relative to a base of 77°F (15°C).
FIGURE 4 Gas-turbine flow diagram.
The air may be treated in calculations as a perfect gas with a constant-
pressure-specific heat of 0.24 Btu/(lb. °F) [1.005 kJ/(kg. ° C)]. The products
of combustion have an enthalpy of 15,400 Btu/lb · mol) [33,950 Btu/(kg ·
mol)] at 1600°F (871°C) and an enthalpy of 3750 Btu/(lb · mol) [8270
Btu/(kg · mol)] at 77°F (24°C). Determine, assuming complete combustion
and neglecting dissociation, the required air temperature at the inlet of the
combustion chamber.
Calculation Procedure:
1. Find the amount of oxygen required for complete combustion of the fuel
Eight atoms of carbon in C combine with 8 molecules of oxygen, O , and
2
8