Page 181 - Materials Chemistry, Second Edition
P. 181
Part 2b: Operational annex 167
4.3.1 Depletion of abiotic resources
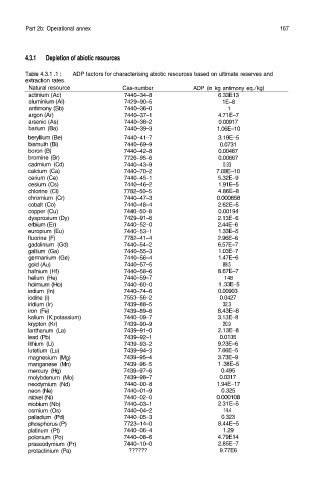
Table 4.3.1 .1 : ADP factors for characterising abiotic resources based on ultimate reserves and
extraction rates.
Natural resource Cas-number ADP (in kg antimony eq./ kg)
actinium (Ac) 7440–34–8 6.33E13
aluminium (Al) 7429–90–5 1E–8
antimony (Sb) 7440–36–0 1
argon (Ar) 7440–37–1 4.71E–7
arsenic (As) 7440–38–2 0.00917
barium (Ba) 7440–39–3 1.06E–10
beryllium (Be) 7440–41–7 3.19E–5
bismuth (Bi) 7440–69–9 0.0731
boron (B) 7440–42–8 0.00467
bromine (Br) 7726–95–6 0.00667
cadmium (Cd) 7440–43–9 0.33
calcium (Ca) 7440–70–2 7.08E–10
cerium (Ce) 7440–45–1 5.32E–9
cesium (Cs) 7440–46–2 1.91E–5
chlorine (Cl) 7782–50–5 4.86E–8
chromium (Cr) 7440–47–3 0.000858
cobalt (Co) 7440–48–4 2.62E–5
copper (Cu) 7440–50–8 0.00194
dysprosium (Dy) 7429–91–6 2.13E–6
erbium (Er) 7440–52–0 2.44E–6
europium (Eu) 7440–53–1 1.33E–5
fluorine (F) 7782–41–4 2.96E–6
gadolinium (Gd) 7440–54–2 6.57E–7
gallium (Ga) 7440–55–3 1.03E–7
germanium (Ge) 7440–56–4 1.47E–6
gold (Au) 7440–57–5 89.5
hafnium (Hf) 7440–58–6 8.67E–7
helium (He) 7440–59–7 148
holmium (Ho) 7440–60–0 1 .33E–5
indium (In) 7440–74–6 0.00903
iodine (I) 7553–56–2 0.0427
iridium (Ir) 7439–88–5 32.3
iron (Fe) 7439–89–6 8.43E–8
kalium (K;potassium) 7440–09–7 3.13E–8
krypton (Kr) 7439–90–9 20.9
lanthanum (La) 7439–91–0 2.13E–8
lead (Pb) 7439–92–1 0.0135
lithium (Li) 7439–93–2 9.23E–6
lutetium (Lu) 7439–94–3 7.66E–5
magnesium (Mg) 7439–95–4 3.73E–9
manganese (Mn) 7439–96–5 1 .38E–5
mercury (Hg) 7439–97–6 0.495
molybdenum (Mo) 7439–98–7 0.0317
neodymium (Nd) 7440–00–8 1.94E–17
neon (Ne) 7440–01–9 0.325
nickel (Ni) 7440–02–0 0.000108
niobium (Nb) 7440–03–1 2.31E–5
osmium (Os) 7440–04–2 14.4
palladium (Pd) 7440–05–3 0.323
phosphorus (P) 7723–14–0 8.44E–5
platinum (Pt) 7440–06–4 1.29
polonium (Po) 7440–08–6 4.79E14
praseodymium (Pr) 7440–10–0 2.85E–7
protactinium (Pa) ?????? 9.77E6