Page 164 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 164
Cathodes 141
It is well known that oxidation/ corrosion of metallic materials is
enhanced [63] in the presence of water vapour. Chromium poisoning can also
be enhanced in the presence of water vapour. The vapour pressure of chromium
containing species increases with increasing water vapour pressure, because
Cr02(0H)2 is relatively stable species in the presence of water vapour [64]. Its
vapour pressure shows only a small temperature dependence so that even at
low temperatures its vapour pressure is high. Taniguchi et aI. found that
the degradation increases with water vapour pressure consistent with the
increasing vapour pressure of Cr-containing species.
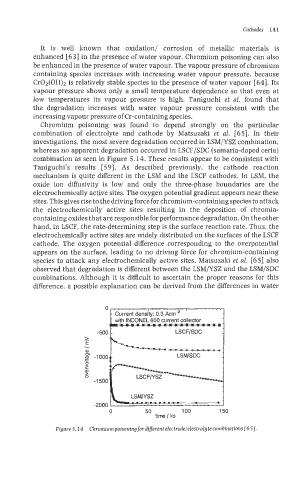
Chromium poisoning was found to depend strongly on the particular
combination of electrolyte and cathode by Matsuzaki et al. [65]. In their
investigations, the most severe degradation occurred in LSM/YSZ combination,
whereas no apparent degradation occurred in LSCF/SDC (samaria-doped ceria)
combination as seen in Figure 5.14. These results appear to be consistent with
Taniguchi’s results [ 5 91. As described previously, the cathode reaction
mechanism is quite different in the LSM and the LSCF cathodes. In LSM, the
oxide ion diffusivity is low and only the three-phase boundaries are the
electrochemically active sites. The oxygen potential gradient appears near these
sites. This gives rise to the driving force for chromium-containing species to attack
the electrochemically active sites resulting in the deposition of chromia-
containing oxides that are responsible for performance degradation. On the other
hand, in LSCF, the rate-determining step is the surface reaction rate. Thus, the
electrochemically active sites are widely distributed on the surfaces of the LSCF
cathode. The oxygen potential difference corresponding to the overpotential
appears on the surface, leading to no driving force for chromium-containing
species to attack any electrochemically active sites. Matsuzaki et al. [65] also
observed that degradation is different between the LSM/YSZ and the LSM/SDC
combinations. Although it is difficult to ascertain the proper reasons for this
difference, a possible explanation can be derived from the differences in water
-500 LSCF/SDC
0 50 100 150
time I ks
Figure 5.24 Chromiumpoisoningfor different electrode/electrolyte combinations 1651.