Page 254 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 254
Electrode Polarisations 23 1
the local current density. Thus, the Nernst voltage, E, is not a function of current
density. This assumption is valid only if the flow rates of fuel and oxidant are
sufficiently high such that the fuel and oxidant compositions just outside of the
anode and cathode, respectively, are virtually fixed. If this is not the case, then
the OCV itself must be treated as a function of current density. The dependence of
E on current density can be estimated assuming the respective cathodic and
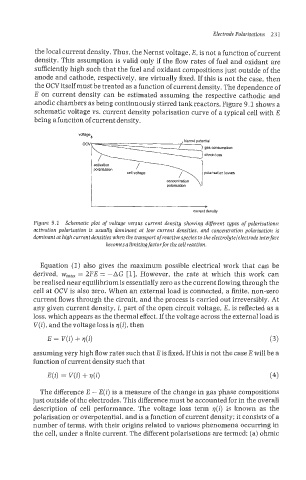
anodic chambers as being continuously stirred tank reactors. Figure 9.1 shows a
schematic voltage vs. current density polarisation curve of a typical cell with E
being a function of current density.
voltage
Nemst potential
activation
polarisation
polansation
current density
Figure 9.1 Schematic plot of voltage versus current density showing different types of polarisations:
activation polarisation is usually dominant at low current densities, and concentration polarisation is
dominant at high current densities when the transport of reactive species to the electrolytelelectrode interface
becomes alimiting factorfor the cell reaction.
Equation (1) also gives the maximum possible electrical work that can be
derived, wmax = 2FE = -AG [l]. However, the rate at which this work can
be realised near equilibrium is essentially zero as the current flowing through the
cell at OCV is also zero. When an external load is connected, a finite, non-zero
current flows through the circuit, and the process is carried out irreversibly. At
any given current density, i, part of the open circuit voltage, E, is reflected as a
loss, which appears as the thermal effect. If the voltage across the external load is
V(i), and the voltage loss is q(i), then
E = V(i) + q(i) (3)
assuming very high flow rates such that E is fixed. If this is not the case E will be a
function of current density such that
E(i) = V(i) + q(i) (4)
The difference E - E(i) is a measure of the change in gas phase compositions
just outside of the electrodes. This difference must be accounted for in the overall
description of cell performance. The voltage loss term q(i) is known as the
polarisation or overpotential, and is a function of current density: it consists of a
number of terms, with their origins related to various phenomena occurring in
the cell, under a finite current. The different polarisations are termed: (a) ohmic