Page 63 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 63
History 41
to construction of a working SOFC were the cathode material and the
electronically conducting interconnection material, together with the problems
of suitable fabrication techniques for producing gastight thin films of electrolyte
and interconnection, especially in their overlap regions [12 71.
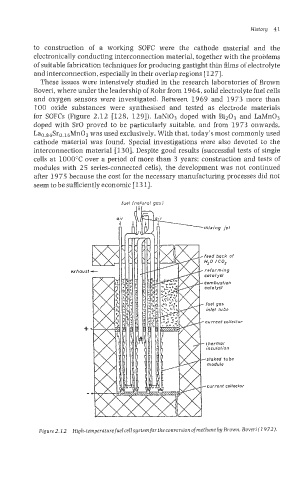
These issues were intensively studied in the research laboratories of Brown
Boveri, where under the leadership of Rohr from 1964, solid electrolyte fuel cells
and oxygen sensors were investigated. Between 1969 and 1973 more than
100 oxide substances were synthesised and tested as electrode materials
for SOFCs (Figure 2.12 [128, 1291). LaNi03 doped with Bi203 and LaR/ln03
doped with SrO proved to be particularly suitable, and from 1973 onwards,
Lao.s4Sro.16Mn03 was used exclusively. With that, today’s most commonly used
cathode material was found. Special investigations were also devoted to the
interconnection material [I 301. Despite good results (successful tests of single
cells at 1000°C over a period of more than 3 years: construction and tests of
modules with 2 5 series-connected cells), the development was not continued
after 19 75 because the cost for the necessary manufacturing processes did not
seem to be sufficiently economic [ 13 11.
fuel (nolural gas;
.*.
exhausl
+
Figure 2.22 High-temperaturefuelcelIsystenzfor the conversion ofmethane by Brown, Boveri (I 972).