Page 152 - Hydrocarbon
P. 152
Reservoir Description 139
6.2.9.1. Capillary pressure
Returning to the experiment with the oil, water and the glass capillaries (Figure 6.27),
the interfacial tension and wettability lead to a pressure differential across the liquid
interface and a contact angle with the glass. The pressure in the water phase is greater
than the pressure in the oil phase, and the glass is water wet, as determined by the
contactangle.Thepressuredifferencebetween the water phaseand theoil phaseis
called the capillary pressure (P c ), and is related to the interfacial tension (s), the radius of
the capillary tube (r t ) and the contact angle (y)by
2s cos y
P c ¼
r t
Notice that the capillary pressure is greater for smaller capillaries (or throat sizes),
and that when the capillary has an infinite radius, as on the outside of the capillaries
in the tray of water, P c is zero.
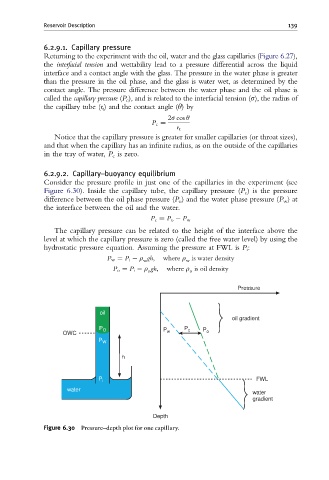
6.2.9.2. Capillary–buoyancy equilibrium
Consider the pressure profile in just one of the capillaries in the experiment (see
Figure 6.30). Inside the capillary tube, the capillary pressure (P c ) is the pressure
difference between the oil phase pressure (P o ) and the water phase pressure (P w )at
the interface between the oil and the water.
P c ¼ P o P w
The capillary pressure can be related to the height of the interface above the
level at which the capillary pressure is zero (called the free water level) by using the
hydrostatic pressure equation. Assuming the pressure at FWL is P i :
P w ¼ P i r gh; where r is water density
w w
P o ¼ P i r gh; where r is oil density
o o
Pressure
oil
oil gradient
P O P P c P
OWC w o
P W
h
P i FWL
water
water
gradient
Depth
Figure 6.30 Pressure^depth plot for one capillary.