Page 166 - Industrial Ventilation Design Guidebook
P. 166
I 28 CHAPTER 4 PHYSICAL FUNDAMENTALS
giving
Integrating Eq. (4.242) gives
where z is the thickness of the diffusion layer, c A2 = C A(Z = z), and
C
A\ = C A(Z = 0).
By giving j A a constant value, C A(Z) can be calculated from Eq. (4.243) for
different z values. The concentration C B can then be calculated as C B(Z) —
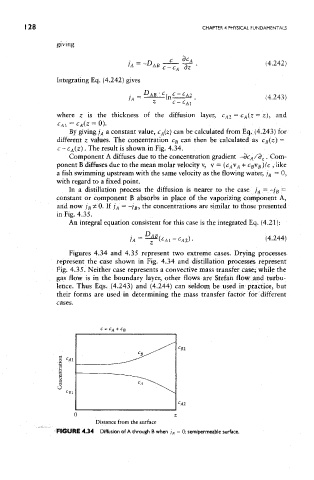
c - C A(Z) . The result is shown in Fig. 4.34.
Component A diffuses due to the concentration gradient -dc A/d z. Com-
ponent B diffuses due to the mean molar velocity v, v = ( C AV A + C BV B )lc , like
a fish swimming upstream with the same velocity as the flowing water, ; B = 0,
with regard to a fixed point.
In a distillation process the diffusion is nearer to the case j A — -j B =
constant or component B absorbs in place of the vaporizing component A,
and now j B *• 0. If j A = —j B, the concentrations are similar to those presented
in Fig. 4.35.
An integral equation consistent for this case is the integrated Eq. (4.21):
Figures 4.34 and 4.35 represent two extreme cases. Drying processes
represent the case shown in Fig. 4.34 and distillation processes represent
Fig. 4.35. Neither case represents a convective mass transfer case; while the
gas flow is in the boundary layer, other flows are Stefan flow and turbu-
lence. Thus Eqs. (4.243) and (4.244) can seldom be used in practice, but
their forms are used in determining the mass transfer factor for different
cases.
FIGURE 4.34 Diffusion of A through B when j B = 0: semipermeable surface.