Page 80 - Industrial Ventilation Design Guidebook
P. 80
4.i FLUID FLOW 45
It is the dynamic viscosity /x of the gas/fluid that determines its ability for
free flow. Very viscous fluids require a large energy input to overcome the trie-
tional forces.
4. i. i .2 Properties of Fluids
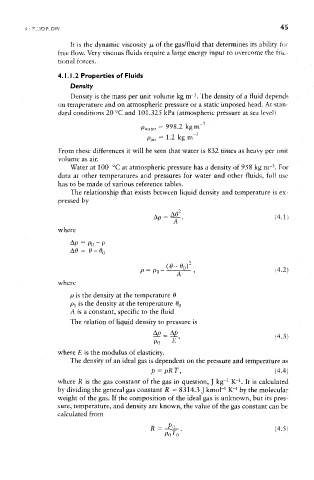
Density
3
Density is the mass per unit volume kg irr . The density of a fluid depends
on temperature and on atmospheric pressure or a static imposed head. At stan-
dard conditions 20 °C and 101.325 kPa (atmospheric pressure at sea level)
From these differences it will be seen that water is 832 times as heavy per unit
volume as air.
3
Water at 100 °C at atmospheric pressure has a density of 958 kg m~ . For
data at other temperatures and pressures for water and other fluids,, full use
has to be made of various reference tables.
The relationship that exists between liquid density and temperature is ex-
pressed by
where
A i
where
p is the density at the temperature 6
Po is the density at the temperature 0 0
A is a constant, specific to the fluid
The relation of liquid density to pressure is
where £ is the modulus of elasticity.
The density of an ideal gas is dependent on the pressure and temperature as
1
1
where R is the gas constant of the gas in question, J kg" Kr . It is calculated
1
by dividing the general gas constant R — 8314.3 J kmoH Kr by the molecular
weight of the gas. If the composition of the ideal gas is unknown, but its pres-
sure, temperature, and density are known, the value of the gas constant can be
calculated from