Page 322 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 322
Multiple-Coll~~tor ICP-MS 307
0,9149
0.91 47
23
a
0.91 45
0.9143
2.1680
2.1670
2.1660
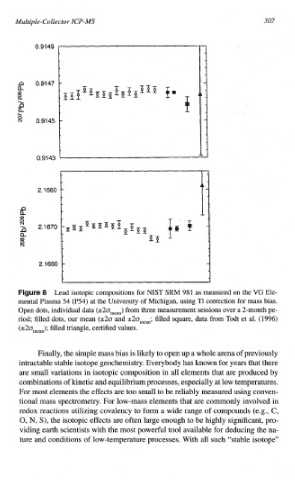
Lead isotopic compositions for NIST SW 981 as measured on the VG Ele-
mental Plasma 54 (P54) at the University of Michigan, using T1 correction for mass bias.
Open dots, individual data (rt20mean) from three ~easure~ent sessions over a 2-month pe-
riod; filled dots, our mean (k2o and rt20mean; filled square, data from Todt et al. (1996)
(rt20mean); filled triangle, certified values.
Finally, the simple mass bias likely to open up a whole arena of previously
is
intractable stable isotope geochemistry. Everybody has known for years that there
are small variations in isotopic composition in all elements that are produced by
combinations of kinetic and equilibrium processes, especially at low temperatures.
For most elements the effects are too small to be reliably measured using conven-
tional mass spectrometry. For low-mass elements that are commonly involved in
redox reactions utilizing covalency to form a wide range of compounds (e.g., C,
0, N, S), the isotopic effects are often large enough to be highly significant, pro-
viding earth scientists with the most powerful tool available for deducing the na-
ture and conditions of low-temperature processes. With all such “stable isotope”