Page 16 - Inorganic Mass Spectrometry : Fundamentals and Applications
P. 16
6 S~it~
where q is the electrochemical potential and includes both the electrostatic and
chemical parts of the work involved in the transition of atom to ion. The proba-
bility that the state is not occupied is given by
W+ = 1 - VV(Ec) = (1 + exp [-(ITc - q)/kTj}-i
The charge transfer probability for an atom is proportional to the ratio of these
probabilities:
W+ 1 + exp [(Ec - q)/kt
-=A{ = A exp [(Ec - qO/kt
WO 1 + exp [-(Ec - q)kt
A is the ratio of statistical weights g+/gO defined previously [Eq. (1.5)]. In Eq.
(1.8), Ec - q = a? - IrC where Ire is the ionization potential for the adsorbed atom
at distance rc from the surface. Each of these expressions defines the change in
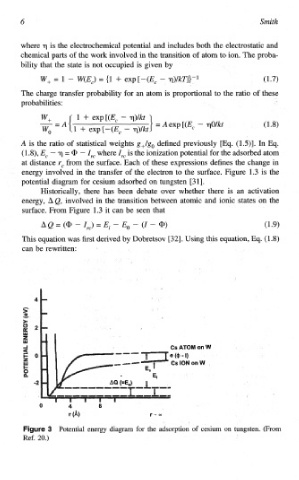
energy involved in the transfer of the electron to the surface. Figure 1.3 is the
potential diagram for cesium adsorbed on tungsten [3 11.
Historically, there has been debate over whether there is an activation
energy, AQ, involved in the transition between atomic and ionic states on the
surface. From Figure 1.3 it can be seen that
AQ = (a? - Ire) = Ei - EO - (I - a?) (1.9)
This equqtion was first derived by Dobretsov [32], Using this equation, Eq. (1 '8)
can be rewritten:
4
i
Cs ATOM on W
0
W
t==
2
-2
1 1 1 1 1
0 4 8
r (A)
Potential energy diagram for the adsorption of cesium on tungsten. (From
Ref. 20.)