Page 100 - Instant notes
P. 100
Physical Chemistry 86
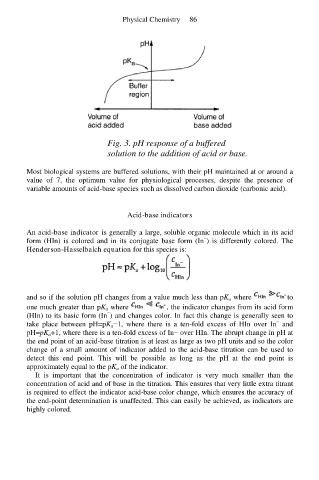
Fig. 3. pH response of a buffered
solution to the addition of acid or base.
Most biological systems are buffered solutions, with their pH maintained at or around a
value of 7, the optimum value for physiological processes, despite the presence of
variable amounts of acid-base species such as dissolved carbon dioxide (carbonic acid).
Acid-base indicators
An acid-base indicator is generally a large, soluble organic molecule which in its acid
−
form (HIn) is colored and in its conjugate base form (In ) is differently colored. The
Henderson-Hasselbalch equation for this species is:
and so if the solution pH changes from a value much less than pK a where to
one much greater than pK a where , the indicator changes from its acid form
−
(HIn) to its basic form (In ) and changes color. In fact this change is generally seen to
−
take place between pH=pK a−1, where there is a ten-fold excess of HIn over In and
pH=pK a+1, where there is a ten-fold excess of In− over HIn. The abrupt change in pH at
the end point of an acid-base titration is at least as large as two pH units and so the color
change of a small amount of indicator added to the acid-base titration can be used to
detect this end point. This will be possible as long as the pH at the end point is
approximately equal to the pK a of the indicator.
It is important that the concentration of indicator is very much smaller than the
concentration of acid and of base in the titration. This ensures that very little extra titrant
is required to effect the indicator acid-base color change, which ensures the accuracy of
the end-point determination is unaffected. This can easily be achieved, as indicators are
highly colored.