Page 323 - Instant notes
P. 323
I2
PRACTICAL ASPECTS OF
SPECTROSCOPY
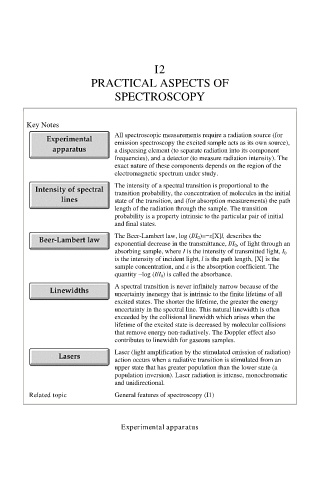
Key Notes
All spectroscopic measurements require a radiation source (for
emission spectroscopy the excited sample acts as its own source),
a dispersing element (to separate radiation into its component
frequencies), and a detector (to measure radiation intensity). The
exact nature of these components depends on the region of the
electromagnetic spectrum under study.
The intensity of a spectral transition is proportional to the
transition probability, the concentration of molecules in the initial
state of the transition, and (for absorption measurements) the path
length of the radiation through the sample. The transition
probability is a property intrinsic to the particular pair of initial
and final states.
The Beer-Lambert law, log (I/I 0 )=−ε[X]l, describes the
exponential decrease in the transmittance, I/I 0 , of light through an
absorbing sample, where I is the intensity of transmitted light, I 0
is the intensity of incident light, l is the path length, [X] is the
sample concentration, and ε is the absorption coefficient. The
quantity −log (I/I 0 ) is called the absorbance.
A spectral transition is never infinitely narrow because of the
uncertainty inenergy that is intrinsic to the finite lifetime of all
excited states. The shorter the lifetime, the greater the energy
uncertainty in the spectral line. This natural linewidth is often
exceeded by the collisional linewidth which arises when the
lifetime of the excited state is decreased by molecular collisions
that remove energy non-radiatively. The Doppler effect also
contributes to linewidth for gaseous samples.
Laser (light amplification by the stimulated emission of radiation)
action occurs when a radiative transition is stimulated from an
upper state that has greater population than the lower state (a
population inversion). Laser radiation is intense, monochromatic
and unidirectional.
Related topic General features of spectroscopy (I1)
Experimental apparatus