Page 55 - Instant notes
P. 55
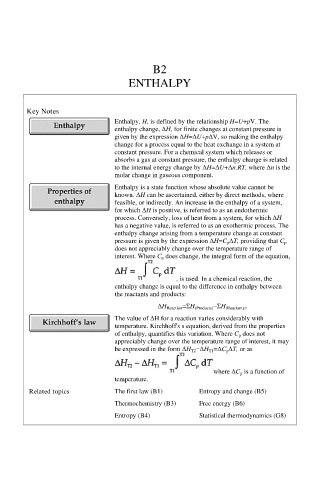
B2
ENTHALPY
Key Notes
Enthalpy, H, is defined by the relationship H=U+pV. The
enthalpy change, ∆H, for finite changes at constant pressure is
given by the expression ∆H=∆U+p∆V, so making the enthalpy
change for a process equal to the heat exchange in a system at
constant pressure. For a chemical system which releases or
absorbs a gas at constant pressure, the enthalpy change is related
to the internal energy change by ∆H=∆U+∆n.RT, where ∆n is the
molar change in gaseous component.
Enthalpy is a state function whose absolute value cannot be
known. ∆H can be ascertained, either by direct methods, where
feasible, or indirectly. An increase in the enthalpy of a system,
for which ∆H is positive, is referred to as an endothermic
process. Conversely, loss of heat from a system, for which ∆H
has a negative value, is referred to as an exothermic process. The
enthalpy change arising from a temperature change at constant
pressure is given by the expression ∆H=C p ∆T, providing that C p
does not appreciably change over the temperature range of
interest. Where C p does change, the integral form of the equation,
, is used. In a chemical reaction, the
enthalpy change is equal to the difference in enthalpy between
the reactants and products:
∆H Reaction =ΣH (Products) −ΣH (Reactants) .
The value of ∆H for a reaction varies considerably with
temperature. Kirchhoff's s equation, derived from the properties
of enthalpy, quantifies this variation. Where C p does not
appreciably change over the temperature range of interest, it may
be expressed in the form ∆H T2 −∆H T1 =∆C p ∆T, or as
where ∆C p is a function of
temperature.
Related topics The first law (B1) Entropy and change (B5)
Thermochemistry (B3) Free energy (B6)
Entropy (B4) Statistical thermodynamics (G8)